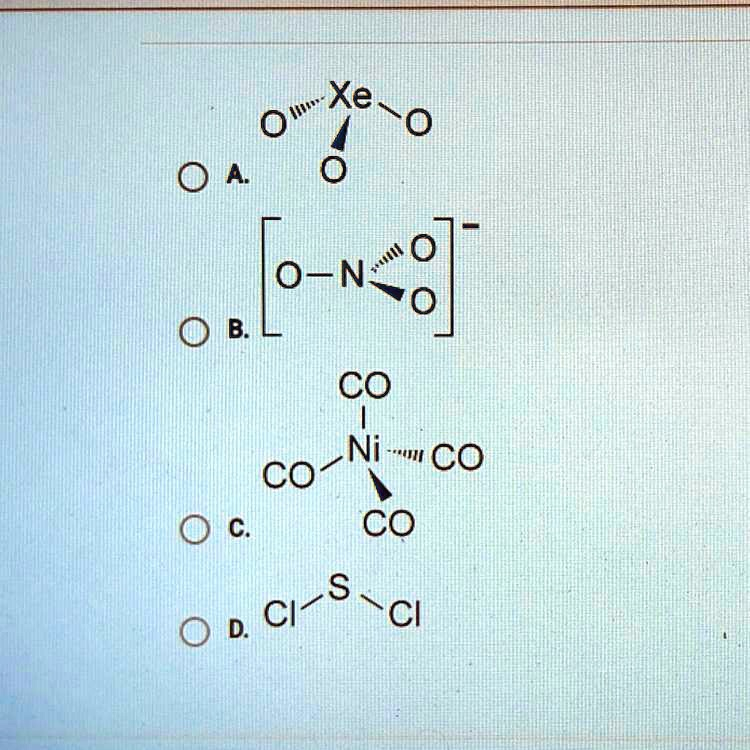
Xe=O A. [O-N=O]^{-} B. CO | CO-Ni-CO CO C. Cl-S-Cl D.
The Correct Answer and Explanation is:
The correct answer is C. CO | CO–Ni–CO CO, which represents nickel tetracarbonyl, Ni(CO)₄.
Nickel tetracarbonyl is a neutral, volatile organometallic compound where a nickel(0) center is bonded to four carbon monoxide ligands through coordinate covalent bonds. Each CO ligand donates a lone pair from its carbon atom to the vacant orbitals on nickel. This forms a tetrahedral geometry with 109.5° bond angles, minimizing electron pair repulsion and contributing to the compound’s stability.
This molecule is of particular interest in inorganic and organometallic chemistry. It is diamagnetic and adheres to the 18-electron rule, which provides additional thermodynamic stability. Nickel in its zero oxidation state gains 8 electrons from the four CO ligands, completing its valence shell. The bonding involves both σ-donation from CO to nickel and π-backbonding from filled metal d-orbitals into the vacant π* antibonding orbitals of CO. This synergistic interaction weakens the C–O bond within each ligand but strengthens the overall metal-ligand bond.
Ni(CO)₄ is industrially significant in the Mond process, where it facilitates the purification of nickel. It is also important in catalysis and coordination chemistry as a prototype for studying metal–ligand interactions.
Let us briefly consider why the other options are not correct:
A. [O–N=O]⁻ refers to the nitrate ion, but this simplified representation overlooks its resonance structures and planar geometry. The nitrate ion is best described as a hybrid of multiple forms.
B. Xe=O is ambiguous, as xenon typically forms oxides like XeO₃ or XeO₄, not a single double bond to oxygen.
D. Cl–S–Cl refers to sulfur dichloride, which is indeed a real compound with a bent shape, but it lacks the coordination complexity and ligand field interactions present in nickel tetracarbonyl.
Thus, Ni(CO)₄ stands out as the most structurally and chemically sophisticated option.
