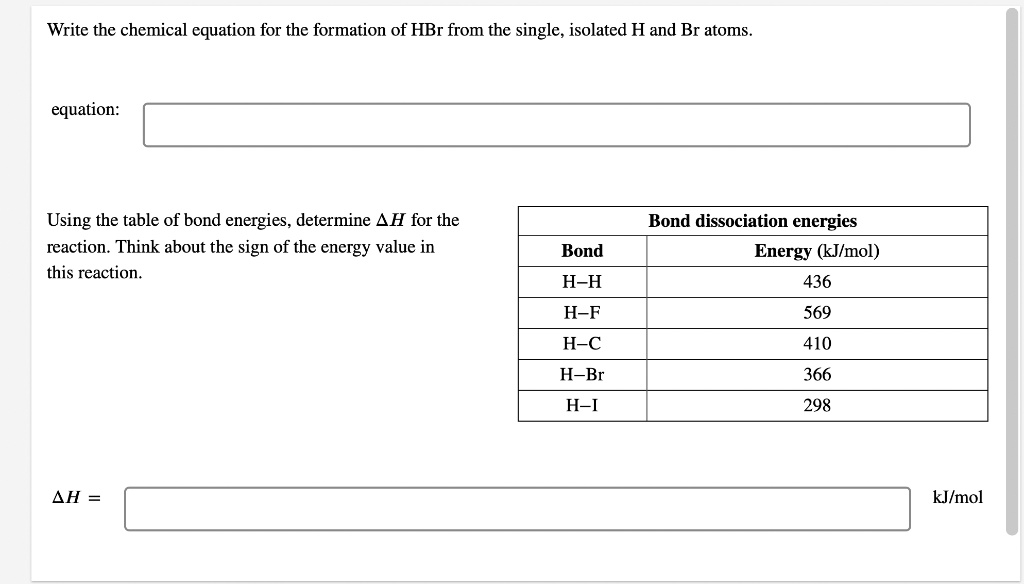
Write the chemical equation for the formation of HBr from the single, isolated H and Br atoms. Equation: H2 + Br2 -> 2HBr Using the table of bond energies, determine ΔH for the reaction. Think about the sign of the energy value in this reaction. Bond dissociation energies Energy (kJ/mol): H-H: 436 H-F: 569 H-C: 410 H-Br: 366 H-I: 298 ΔH (kJ/mol):
The Correct Answer and Explanation is:
The balanced chemical equation for the formation of hydrogen bromide is:
H₂ + Br₂ → 2HBr
To determine the enthalpy change (ΔH) of this reaction, we apply the bond energy method. This involves calculating the total energy required to break bonds in the reactants and subtracting the total energy released during the formation of bonds in the products.
Step 1: Break bonds in the reactants
- Breaking 1 mol of H–H bonds requires 436 kJ
- Breaking 1 mol of Br–Br bonds (not listed, but commonly known) requires 193 kJ
- Total energy input = 436 + 193 = 629 kJ
Step 2: Form bonds in the products
- Forming 2 mol of H–Br bonds releases 2 × 366 = 732 kJ
Step 3: Calculate ΔH
- ΔH = Energy to break bonds − Energy to form bonds
- ΔH = 629 − 732 = −103 kJ/mol
This negative enthalpy change means the reaction is exothermic. More energy is released in forming the H–Br bonds than is absorbed to break the H–H and Br–Br bonds.
Explanation: In a chemical reaction, bond energies can be used to estimate the heat change by considering bonds broken and formed. Bonds must be broken in the reactants before new bonds are formed in the products. Breaking bonds always requires energy, whereas forming bonds releases energy. If more energy is released than absorbed, the reaction gives off heat.
Here, it takes 629 kJ/mol to break the original bonds in one mole of hydrogen and one mole of bromine molecules. However, the formation of two moles of hydrogen bromide releases 732 kJ/mol. The result is a net release of 103 kJ of energy per mole of reaction, so the system becomes more stable after the reaction has occurred.
