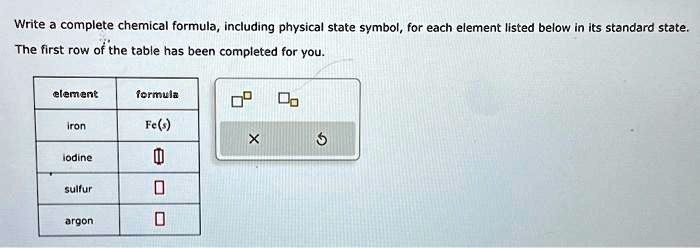
Write a complete chemical formula, including physical state symbol, for each element listed below in its standard state. The first row of the table has been completed for you. element formula iron Fe(s) iodine sulfur argon
The Correct Answer and Explanation is:
Here’s the completed table with chemical formulas and physical state symbols for each element in its standard state:
| Element | Formula |
|---|---|
| Iron | Fe(s) |
| Iodine | I₂(s) |
| Sulfur | S₈(s) |
| Argon | Ar(g) |
Explanation:
The standard state of an element refers to its most stable physical form at 1 atmosphere of pressure and a temperature of 25 degrees Celsius. This is essential when writing chemical reactions and thermodynamic data, as it provides a consistent reference point.
Iron exists as a solid metal in its most stable form, so its formula is Fe with the physical state symbol (s) indicating solid.
Iodine is one of the halogens that form diatomic molecules in their standard state. Therefore, its molecular form is I₂. Since solid iodine has a characteristic shiny dark-gray appearance and sublimates at room temperature, the state symbol is also (s), indicating it is solid under standard conditions.
Sulfur typically exists in the form of S₈ molecules. These are puckered ring structures consisting of eight sulfur atoms. Elemental sulfur in this form is yellow and solid at room temperature, so we write it as S₈(s).
Argon is a noble gas found in the Earth’s atmosphere. It is monoatomic, meaning it consists of individual atoms, not molecules. It is colorless, odorless, and gaseous under standard conditions. Thus, its standard state formula is Ar(g), with (g) indicating the gaseous physical state.
Understanding these standard forms is important in stoichiometry, thermochemistry, and when evaluating physical and chemical properties of elements. Writing accurate formulas helps ensure consistency in chemical equations and allows precise predictions of matter’s behavior in various reactions
.
