Which structure shows the correct electron arrangement in CCl4
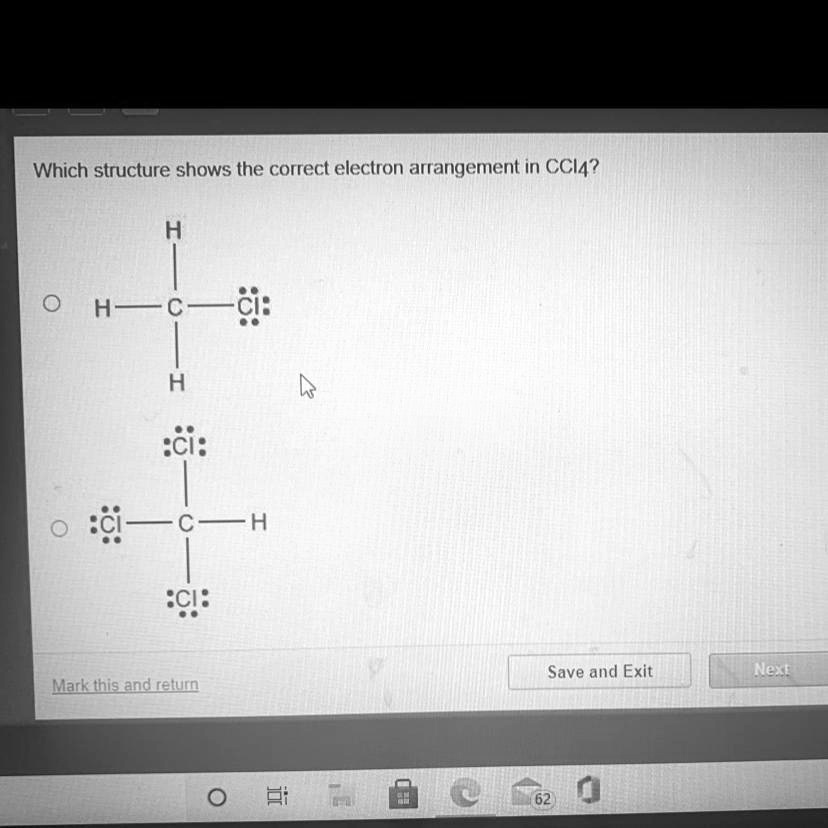
The Correct Answer and Explanation is:
The structure that correctly shows the electron arrangement in carbon tetrachloride (CCl4) is the second option in the image.
Here is the reasoning behind the correct answer:
CCl4 consists of one carbon (C) atom and four chlorine (Cl) atoms. The carbon atom is the central atom, and it forms single bonds with each of the chlorine atoms. Each chlorine atom needs to complete its valence shell with eight electrons, which is achieved by forming a single bond with carbon and having three lone pairs of electrons. On the other hand, the carbon atom has four valence electrons, and by forming four single bonds with the chlorine atoms, it completes its octet.
The correct electron arrangement for CCl4 is represented by the second structure, where:
- The carbon atom is in the center with four single bonds radiating outward to each chlorine atom.
- Each chlorine atom has three lone pairs of electrons, making the total electron count around chlorine correct.
- Carbon has no lone pairs, and the bonding ensures that it also satisfies the octet rule.
In the other options:
- The first structure incorrectly places hydrogen atoms around the carbon atom. Hydrogen cannot bond with chlorine in CCl4.
- The third and fourth structures feature hydrogen bonding to chlorine, which is not part of the CCl4 molecule.
In summary, the second structure is correct because it follows the bonding and electron configuration needed for carbon tetrachloride. Each chlorine atom has its lone pairs, and the central carbon atom satisfies its octet through bonding with chlorine.
