Which of the following molecules should have the highest boiling point
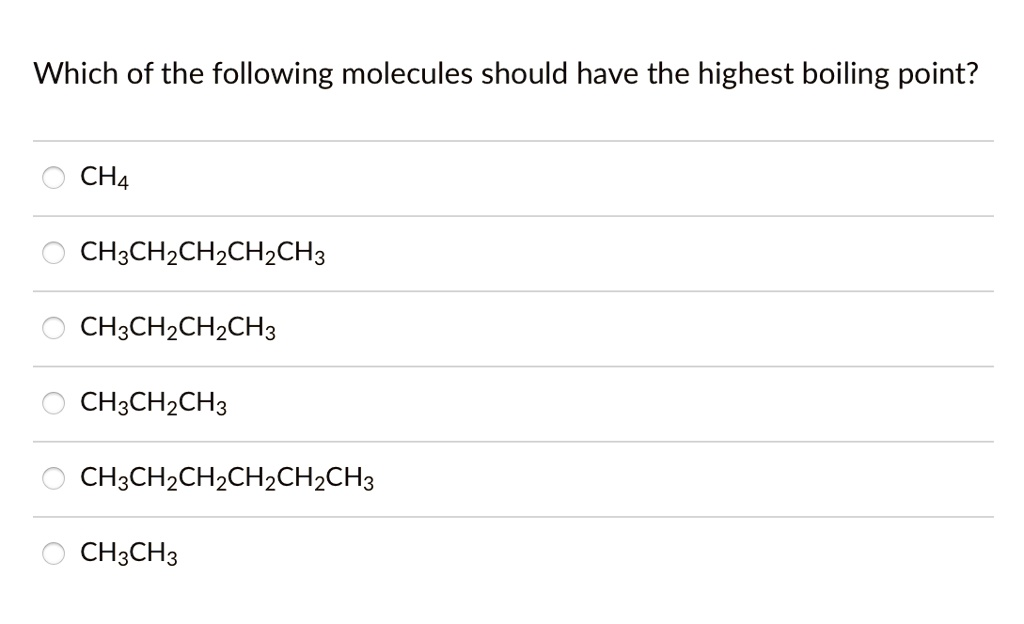
The Correct Answer and Explanation is:
Correct Answer: CH₃CH₂CH₂CH₂CH₂CH₃
Explanation:
The boiling point of a substance is determined by the strength of the intermolecular forces (IMFs) that hold its molecules together in the liquid state. To boil a liquid, enough energy, typically in the form of heat, must be supplied to overcome these attractive forces. Stronger intermolecular forces require more energy to overcome, which translates to a higher boiling point.
All the molecules listed in the question are alkanes. Alkanes are nonpolar hydrocarbons, meaning they consist only of carbon and hydrogen atoms with no significant difference in electronegativity. The only type of intermolecular force present between nonpolar molecules is the London dispersion force. These forces are temporary attractions that occur when the random movement of electrons creates a momentary, instantaneous dipole in one molecule, which then induces a corresponding dipole in a neighboring molecule.
The strength of London dispersion forces depends on two main factors: molecular size (molar mass) and surface area. As the size of a molecule increases, it has more electrons. A larger electron cloud is more easily distorted or polarized, leading to stronger and more frequent temporary dipoles. This results in stronger overall London dispersion forces.
When we examine the given options, we see a series of straight-chain alkanes of increasing length:
- CH₄: Methane (1 carbon)
- CH₃CH₃: Ethane (2 carbons)
- CH₃CH₂CH₃: Propane (3 carbons)
- CH₃CH₂CH₂CH₃: Butane (4 carbons)
- CH₃CH₂CH₂CH₂CH₃: Pentane (5 carbons)
- CH₃CH₂CH₂CH₂CH₂CH₃: Hexane (6 carbons)
Hexane (CH₃CH₂CH₂CH₂CH₂CH₃) is the largest molecule in the list. It has the highest molar mass, the most electrons, and the largest surface area for interaction. Consequently, it experiences the strongest London dispersion forces among all the choices. Because more energy is needed to overcome these stronger attractions, hexane has the highest boiling point.
