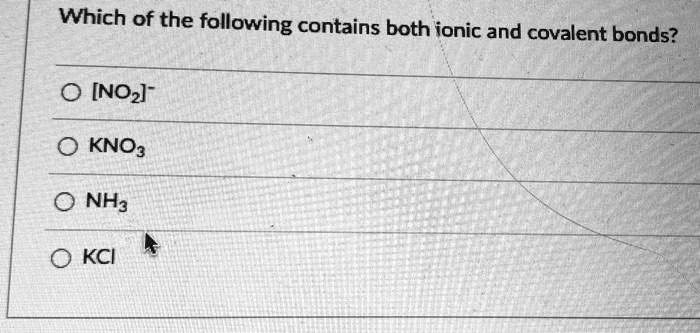
Which of the following contains both ionic and covalent bonds? [NOz]- KNO3 NH3 KCI
The Correct Answer and Explanation is:
The correct answer is KNO3 (potassium nitrate).
Explanation:
KNO3 is a compound that consists of both ionic and covalent bonds. It is composed of potassium ions (K⁺) and nitrate ions (NO3⁻). The bond between potassium and nitrate is ionic, while the bonds within the nitrate ion itself are covalent.
- Ionic bond in KNO3 Potassium, a Group 1 metal, readily loses one electron to form a K⁺ ion. The nitrate ion (NO3⁻) carries a negative charge due to extra electrons. Since opposite charges attract, an ionic bond forms between K⁺ and NO3⁻.
- Covalent bonds in NO3⁻ Within the nitrate ion, nitrogen shares electrons with three oxygen atoms through covalent bonds. Oxygen is highly electronegative and pulls electron density towards itself, making the bonds polar covalent. The nitrate ion has resonance structures, meaning electron delocalization occurs, stabilizing the ion.
Comparison with Other Options:
- [NO2]⁻: Contains only covalent bonds between nitrogen and oxygen.
- NH3: Contains only covalent bonds between nitrogen and hydrogen.
- KCl: Contains only ionic bonds between potassium and chloride.
Importance of Recognizing Both Bond Types:
Understanding compounds with both ionic and covalent bonds is crucial in chemistry. Such compounds often exhibit unique solubility, conductivity, and reactivity patterns. Potassium nitrate, for example, dissolves in water due to its ionic nature but maintains structural stability due to covalent bonding within the nitrate ion.
This knowledge is essential in fields like analytical chemistry, reaction mechanism studies, and even pharmaceutical applications where ionic-covalent interactions play a role in solubility and drug efficacy.
