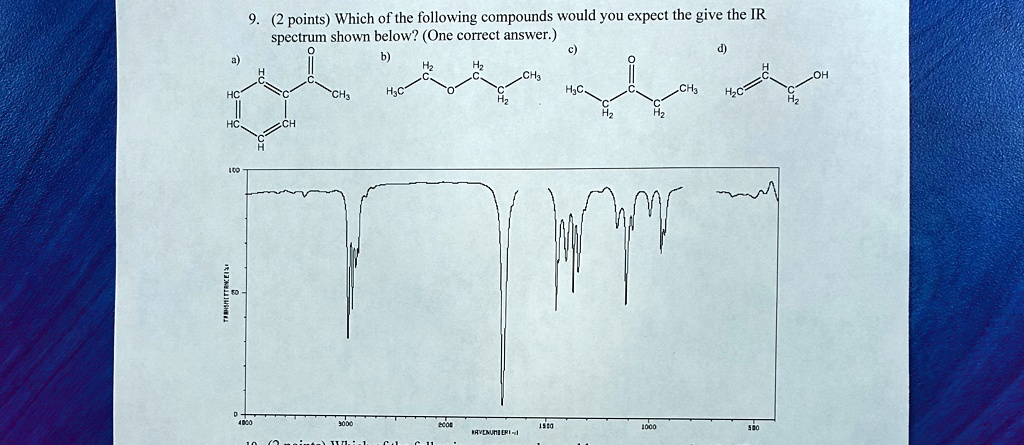
Which of the following compounds would you expect the give the IR spectrum shown below? (One correct answer.) a) \text{Image of a molecule} b) \text{Image of a molecule} c) \text{Image of a molecule} d) \text{Image of a molecule} \text{Image of an IR spectrum}
The Correct Answer and Explanation is:
The compound that corresponds to the given IR spectrum is compound c.
To justify this choice, let’s analyze the key absorption features:
- Sharp, strong peak near 1700 cm⁻¹ This is characteristic of a carbonyl (C=O) stretching vibration, which appears in the range 1680–1750 cm⁻¹. All compounds except b and d contain a carbonyl group, immediately eliminating them from consideration.
- Absence of a broad O–H stretch near 3200–3600 cm⁻¹ A broad peak in this region would suggest an alcohol or phenol group. Compounds b and d contain hydroxyl groups, but the spectrum shows no such broad absorption. Therefore, these two can be ruled out.
- No aromatic overtones near 1600–2000 cm⁻¹ and no characteristic peaks near 1500–1600 cm⁻¹ Aromatic rings tend to give multiple moderate peaks in this region. Compound a has a benzene ring, which should generate those signals. Since the spectrum lacks these, compound a does not match.
Now we are left with compound c. It features a carbonyl group (likely a ketone) and two ethyl chains. The IR spectrum supports this because:
- The strong C=O stretch near 1700 cm⁻¹ confirms the presence of a ketone
- The absence of broad O–H stretches rules out alcohols or carboxylic acids
- The fingerprint region (below 1500 cm⁻¹) displays patterns that are typical of saturated hydrocarbons, consistent with ethyl groups
In conclusion, compound c matches the IR spectrum due to the presence of a ketone group and aliphatic chains, without any hydroxyl or aromatic features. The IR evidence supports this structure better than any of the others presented.
