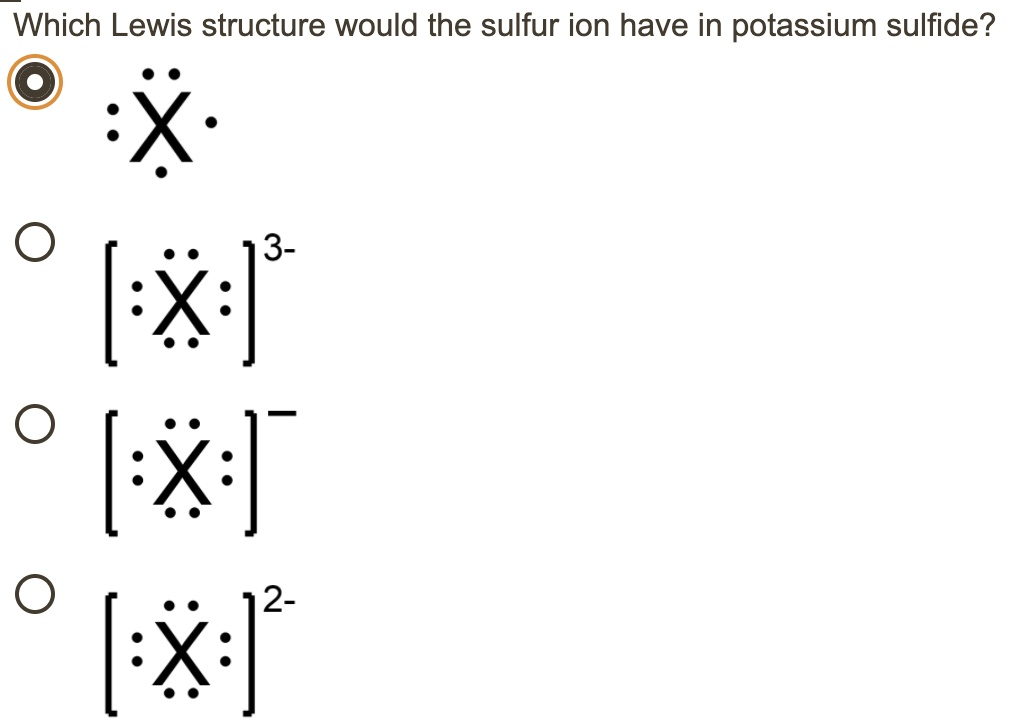
Which Lewis structure would the sulfur ion have in potassium sulfide
The Correct Answer and Explanation is:
The correct Lewis structure for the sulfur ion in potassium sulfide is the one showing sulfur with eight valence electrons and a 2⁻ charge.
Let’s walk through the reasoning. Potassium sulfide, K₂S, consists of two potassium atoms and one sulfur atom. Each potassium atom has a single electron in its outer shell, which it readily loses to form a K⁺ ion. Sulfur, belonging to group 16 of the periodic table, naturally has six valence electrons and needs two more to complete its octet. By accepting one electron from each potassium atom, the sulfur atom achieves a full octet, becoming a sulfide ion, S²⁻.
The Lewis structure of an ion represents its valence electrons, typically depicted as dots around the chemical symbol. For the S²⁻ ion, the structure will show eight dots, representing a complete outer shell, enclosed in brackets with a 2⁻ superscript to indicate its charge. The gain of two electrons explains the negative charge. Since the sulfur ion accepts these electrons in an ionic bond with potassium ions, it does not share electrons through covalent bonding, which distinguishes it from molecular Lewis structures.
Analyzing the options from your image, only one shows sulfur with eight dots and a 2⁻ charge, which accurately represents the electron configuration after the transfer. The other options fail for various reasons: one lacks the full octet, while the others misstate the charge, suggesting sulfur gained the wrong number of electrons.
In conclusion, sulfur in potassium sulfide has a Lewis structure that includes eight valence electrons and a 2⁻ charge. This reflects its stable configuration after gaining two electrons from potassium. It is a textbook example of ionic bonding between a metal and a nonmetal, resulting in complete outer shells for both ions involved.
