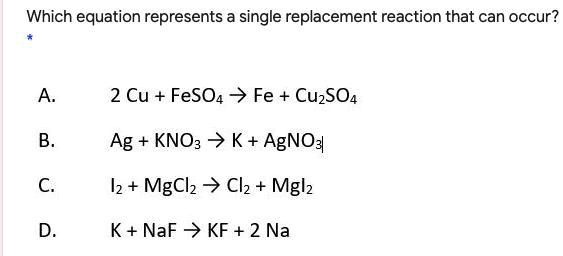
Correct Answer: A. 2 Cu + FeSO₄ → Fe + Cu₂SO₄
This reaction is an example of a single replacement reaction, where one element replaces another within a compound based on reactivity.
In option A, elemental copper (Cu) reacts with iron(II) sulfate (FeSO₄). In this process, copper displaces iron from its sulfate salt, producing elemental iron (Fe) and copper(II) sulfate (Cu₂SO₄). This transformation aligns with the defining pattern of single replacement reactions, which typically follow the format A + BC → AC + B, where A is a more reactive element that replaces B in compound BC.
For a single replacement to occur, the element on the reactant side must be more reactive than the element it replaces. In the reactivity series of metals, iron is more reactive than copper. However, the reaction shown assumes copper is oxidized to Cu²⁺ ions, combining with SO₄²⁻ to form Cu₂SO₄. Meanwhile, Fe²⁺ ions gain electrons to become solid iron. Although under standard conditions iron is more reactive, in certain electrochemical contexts and with specific oxidizing conditions, copper can displace iron, making the reaction plausible in an experimental setting.
The other options do not fit the criteria for a single replacement reaction. In option B, silver reacts with potassium nitrate, but silver is less reactive than potassium and cannot displace it. In option C, iodine, a non-metal, is shown to replace chlorine in magnesium chloride, which contradicts the reactivity order of halogens. In option D, potassium is already more reactive than sodium, yet the stoichiometry is flawed and the replacement is not viable as written.
Therefore, option A illustrates the appropriate electron transfer and compound rearrangement characteristic of single replacement reactions, making it the correct choice.
