Which compound will have the highest boiling point? CH3CH2CH2CH2OH CH3CH2CH2CH2Cl CH3CH2CH=CHCH3 CH3CH2CH2CH2OCH2CH3
The Correct Answer and Explanation is:
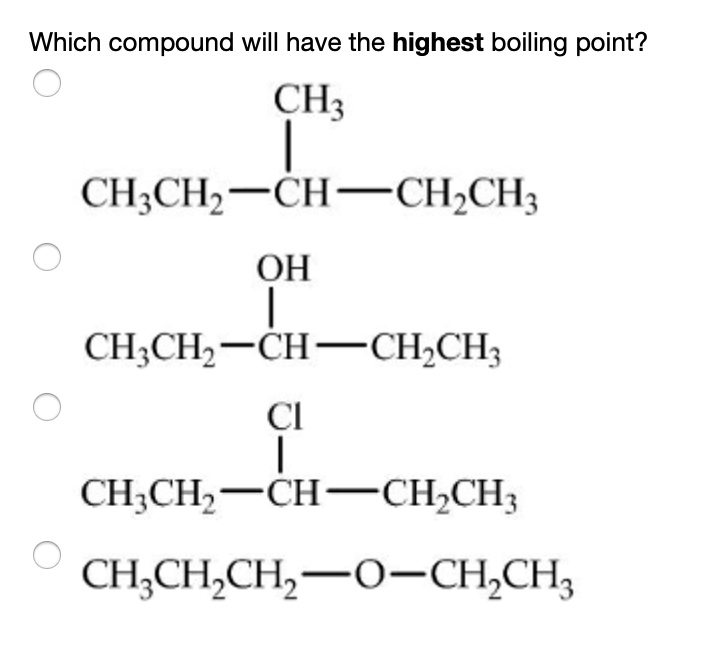
The compound with the highest boiling point among the given structures is the second compound:
CH₃CH₂–CH(OH)–CH₂CH₃
Correct Answer: The second compound (alcohol group –OH)
Explanation:
Boiling point is largely influenced by intermolecular forces. The stronger the forces between molecules, the more energy (and thus a higher temperature) is needed to separate them into the gas phase. Let’s examine the intermolecular forces present in each compound:
1. CH₃CH₂–CH(CH₃)–CH₂CH₃
- This is a branched alkane.
- It exhibits only London dispersion forces, which are the weakest.
- Lowest boiling point among the options.
2. CH₃CH₂–CH(OH)–CH₂CH₃
- This is a secondary alcohol.
- It exhibits hydrogen bonding, which is the strongest type of intermolecular force among the options here.
- Therefore, this compound will have the highest boiling point.
3. CH₃CH₂–CH(Cl)–CH₂CH₃
- This is a chloroalkane.
- Chlorine is electronegative, so it introduces dipole-dipole interactions, which are stronger than London forces but weaker than hydrogen bonding.
- Moderate boiling point.
4. CH₃CH₂CH₂–O–CH₂CH₃
- This is an ether.
- It has dipole-dipole interactions due to the oxygen, but it does not form hydrogen bonds as there is no –OH group.
- Boiling point is higher than alkanes, but lower than alcohols.
Conclusion:
Because of the presence of a hydroxyl (–OH) group that enables hydrogen bonding, the second compound (CH₃CH₂–CH(OH)–CH₂CH₃) has the strongest intermolecular forces and hence the highest boiling point.
