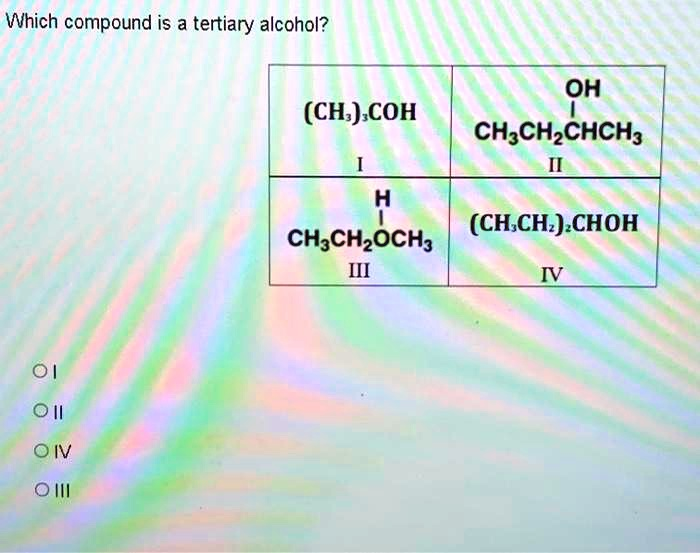
Which compound is a tertiary alcohol? OH (CH3)3COH CH3CH2CHCH3 H (CH3CH2)3CHOH CH3CH2OCH3 MI 01 oil Oil III
The Correct Answer and Explanation is:
To determine which compound is a tertiary alcohol, we must first understand the classification of alcohols. Alcohols are categorized as primary, secondary, or tertiary based on the number of carbon atoms directly bonded to the specific carbon atom that carries the hydroxyl (–OH) group.
A primary (1°) alcohol has the hydroxyl group attached to a carbon atom that is bonded to only one other carbon atom.
A secondary (2°) alcohol has the hydroxyl group attached to a carbon atom bonded to two other carbon atoms.
A tertiary (3°) alcohol has the hydroxyl group attached to a carbon atom that is bonded to three other carbon atoms.
Let’s analyze each compound:
Compound I: (CH₃)₃COH
This formula represents tert-butyl alcohol. The central carbon atom is bonded to the hydroxyl (–OH) group. This same carbon atom is also directly attached to three separate methyl (CH₃) groups. Since the carbon atom bearing the –OH group is attached to three other carbon atoms, Compound I is a tertiary alcohol. Its systematic name is 2-methyl-2-propanol.
Compound II: CH₃CH₂CH(OH)CH₃
This compound is butan-2-ol. The carbon atom carrying the –OH group is part of the main chain. It is bonded to a CH₂ group on its left and a CH₃ group on its right. Therefore, this carbon atom is bonded to two other carbon atoms, which makes Compound II a secondary alcohol.
Compound III: CH₃CH₂OCH₃
This compound is ethyl methyl ether. It does not contain a hydroxyl (–OH) group. Instead, it features an oxygen atom single-bonded between two carbon chains, which is the defining characteristic of an ether functional group. Since it is not an alcohol, it cannot be a tertiary alcohol.
Compound IV: (CH₃CH₂)₂CHOH
This compound is pentan-3-ol. The carbon atom bonded to the –OH group is also attached to two ethyl (CH₃CH₂) groups. This means the hydroxyl-bearing carbon is directly bonded to two other carbon atoms (the CH₂ carbons of the ethyl groups), classifying it as a secondary alcohol.
Based on this analysis, Compound I is the only molecule that fits the definition of a tertiary alcohol
