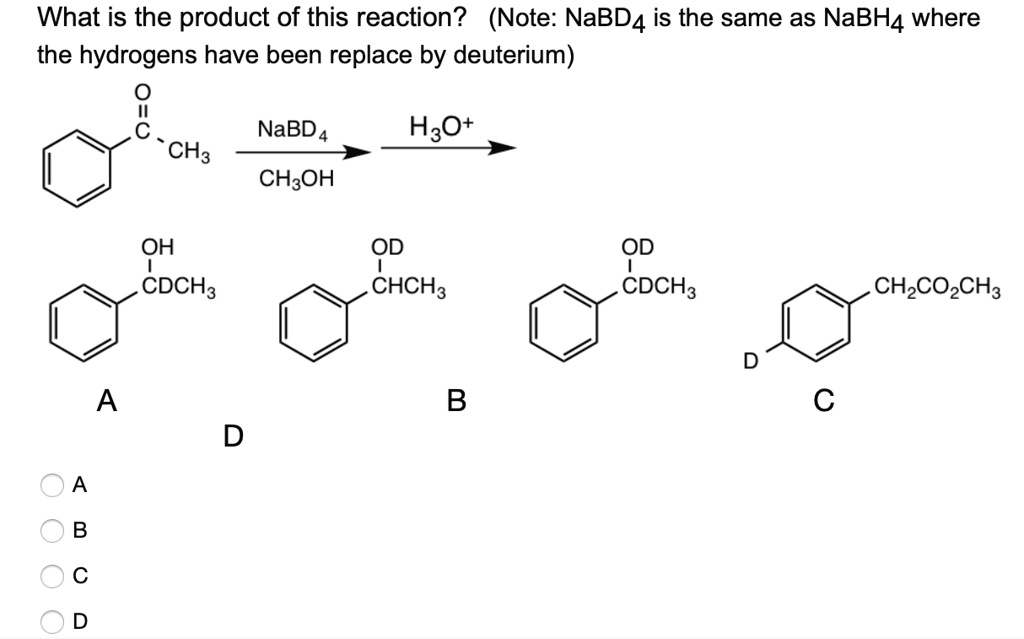
What is the product of this reaction? (Note: NaBD4 is the same as NaBH4 where the hydrogens have been replaced by deuterium) NaBD4 H2O CH3OH OH CDCH3 OD CHCH3 OD CDCH3 CH2CO2CH3 A B CH3
The Correct Answer and Explanation is:
The correct answer is D.
Explanation:
The starting compound is acetophenone, a molecule containing a phenyl group (benzene ring) attached to a carbonyl group (C=O) and a methyl group (CH₃). The reaction involves the use of NaBD₄, which is sodium borodeuteride. This reagent functions like NaBH₄ but donates deuterium (D) instead of hydrogen (H) during reduction.
The reduction of a ketone (like acetophenone) by NaBD₄ occurs in two steps:
- Nucleophilic attack: NaBD₄ donates a deuteride ion (D⁻) to the carbon of the carbonyl group. This breaks the double bond and forms an alkoxide intermediate (Ph-CHD-O⁻).
- Protonation: The alkoxide is then protonated by an acid (H₃O⁺), giving the corresponding alcohol.
As a result, the carbonyl carbon, which was sp² hybridized and double bonded to oxygen, becomes sp³ hybridized and bears both a hydroxyl group (OH) and a deuterium-labeled methyl group (CDH₃). The final product is 1-phenylethanol, but with deuterium at the carbon that was formerly part of the carbonyl group.
Now let’s analyze the options:
- A shows OH and CDCH₃: hydroxyl is present, and a deuterium is on the methyl group, not on the carbon directly attached to the OH, so incorrect.
- B shows OD and CHCH₃: deuterium is incorrectly on the oxygen, not from the hydride donor, so incorrect.
- C is an ester and does not match the starting material, so incorrect.
- D shows OH and CDCH₃: hydroxyl on the former carbonyl carbon, and a deuterium also attached there, which is correct.
Thus, D is the correct product of the reduction of acetophenone using NaBD₄ in methanol followed by acidic work-up.
