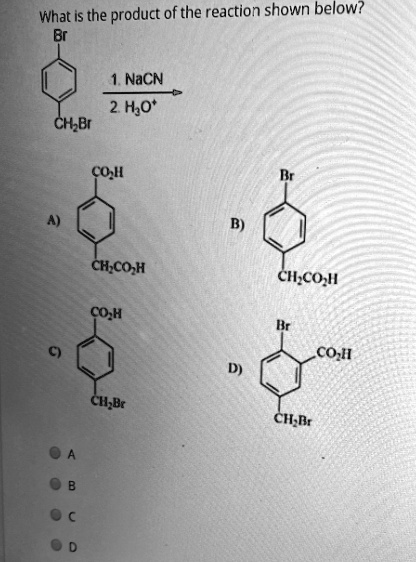
What is the product of the reaction shown below? NaCN + 2 H2O + CHLBr Co II CH3COH CHCOI CoH CoII CHBr CHRt
The Correct Answer and Explanation is:
This is a two-step organic synthesis problem. Let’s analyze the reaction step by step to determine the final product.
Step 1: Reaction with Sodium Cyanide (NaCN)
The starting material is 1-bromo-4-(bromomethyl)benzene. This molecule has two different types of carbon-bromine bonds:
- An aryl bromide, where the bromine is directly attached to the aromatic benzene ring.
- A benzylic bromide, where the bromine is attached to a carbon atom that is adjacent to the benzene ring (-CH₂Br).
Sodium cyanide (NaCN) provides the cyanide ion (CN⁻), which is a good nucleophile. This first step is a nucleophilic substitution reaction. We need to compare the reactivity of the two bromide positions.
- Aryl halides (like the bromine on the ring) are generally unreactive towards nucleophilic substitution (SN2 or SN1 reactions) under standard conditions. The C-Br bond has partial double bond character, and the sp² hybridized carbon makes backside attack for SN2 impossible.
- Benzylic halides (like the -CH₂Br).
Sodium cyanide provides the cyanide ion (CN⁻), which is a strong nucleophile. It will participate in a nucleophilic substitution reaction. Benzylic halides are highly reactive in SN2 reactions because the transition state is stabilized by the adjacent aromatic ring. In contrast, aryl halides are very unreactive towards nucleophilic substitution under these conditions because the C-Br bond has partial double-bond character and the carbon atom is sp² hybridized.
Therefore, the cyanide ion will selectively attack the benzylic carbon, displacing the bromide ion. The aryl bromide will remain unchanged. The intermediate product formed after this first step is 4-bromophenylacetonitrile.
Step 2: Reaction with Hydronium (H₃O⁺)
The second step involves adding acid and water (H₃O⁺), which is a standard procedure for the hydrolysis of a nitrile. The nitrile group (-C≡N) of the intermediate is hydrolyzed to a carboxylic acid group (-COOH). This reaction does not affect the stable aryl bromide bond.
Conclusion
The overall reaction sequence first converts the bromomethyl group (-CH₂Br) into a cyanomethyl group (-CH₂CN) and then hydrolyzes the cyanomethyl group into a carboxymethyl group (-CH₂COOH). The bromine atom on the benzene ring is not affected by either reagent.
The final product is (4-bromophenyl)acetic acid, which corresponds to structure B.
The correct answer is B.Br group) are very reactive in SN2 reactions because the transition state is stabilized by the adjacent aromatic ring.
Therefore, the cyanide ion will selectively attack the more reactive benzylic carbon, displacing the bromide ion. The aryl bromide will remain untouched.
The intermediate product after step 1 is 4-bromophenylacetonitrile.
Reaction: Br-C₆H₄-CH₂Br + NaCN → Br-C₆H₄-CH₂CN + NaBr
Step 2: Reaction with Acid (H₃O⁺)
The second step involves treating the intermediate, a nitrile (R-C≡N), with a strong acid and water (H₃O⁺), typically with heat. This is a standard reaction for the hydrolysis of a nitrile. Acid-catalyzed hydrolysis converts a nitrile group into a carboxylic acid group (-COOH).
The nitrile group (-C≡N) in 4-bromophenylacetonitrile is hydrolyzed to a carboxylic acid group (-COOH). The aryl bromide on the ring is unaffected by these conditions.
Reaction: Br-C₆H₄-CH₂CN + 2H₂O + H⁺ → Br-C₆H₄-CH₂COOH + NH₄⁺
Final Product
The final product of the entire sequence is (4-bromophenyl)acetic acid.
Now, let’s compare this result with the given options:
- A) This product would require both bromides to be substituted and hydrolyzed, which is incorrect.
- B) This is (4-bromophenyl)acetic acid, which matches our derived product.
- C) This product would result from the substitution of the aryl bromide, which is less reactive, leaving the benzylic bromide. This is incorrect.
- D) This product shows a different substitution pattern on the ring, which would not occur in this reaction.
Therefore, the correct product is option B.
The correct answer is B.thumb_upthumb_down
