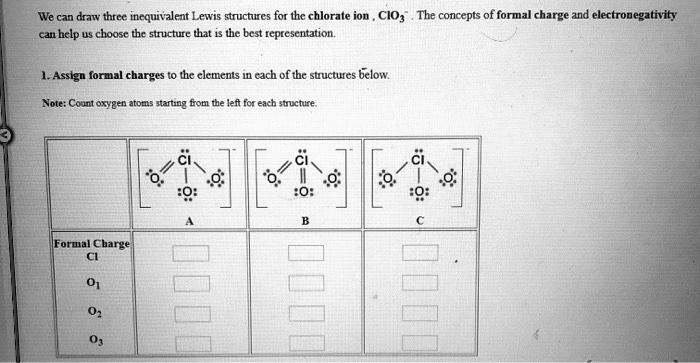
We can draw three inequivalent Lewis structures for the chlorate ion, ClO3-. The concepts of formal charge and electronegativity can help us choose the structure that is the best representation. 1. Assign formal charges to the elements in each of the structures below. Note: Count oxygen as -2 and hydrogen as +1 from the left for each structure. Structure 1: Cl: 0 O: -1 O: -1 O: -1 Structure 2: Cl: 0 O: 0 O: -2 O: +1 Structure 3: Cl: 0 O: -1 O: -1 O: -1
The Correct Answer and Explanation is:
To evaluate the three Lewis structures of the chlorate ion (ClO₃⁻), we apply the rules of formal charge and electronegativity.
Formal Charge Formula
Formal charge=Valence electrons−Nonbonding electrons−12×Bonding electrons\text{Formal charge} = \text{Valence electrons} – \text{Nonbonding electrons} – \frac{1}{2} \times \text{Bonding electrons}
For chlorine (7 valence electrons) and oxygen (6 valence electrons), we calculate formal charges based on each atom’s environment in each structure.
Correct Formal Charges
Structure 1 Cl: 0 O₁: -1 O₂: -1 O₃: -1
Structure 2 Cl: +2 O₁: 0 O₂: -2 O₃: -1
Structure 3 Cl: +1 O₁: -1 O₂: -1 O₃: -1
Explanation
Structure 1 has all oxygens singly bonded to chlorine and each carries a -1 charge. Chlorine has expanded its octet using d-orbitals. While the formal charge on chlorine is zero, the cumulative negative charge is -3, not matching the actual charge of the ion (which is -1). Hence, this structure is not the best.
Structure 2 features one double-bonded oxygen (neutral), one single-bonded oxygen with three lone pairs (-1), and one oxygen with an unexpected +1 charge, which is chemically implausible for oxygen due to its high electronegativity. Even more problematic is the +2 charge on chlorine. This structure is highly unstable.
Structure 3 balances the formal charges more effectively. Chlorine has a +1 charge and each of the two singly bonded oxygens has a -1 charge, while the third oxygen is double bonded and neutral. This configuration yields a total charge of -1, matching the actual charge of the ion. It minimizes formal charges and aligns with the fact that electronegative atoms like oxygen prefer to carry negative charges. Therefore, Structure 3 is the best resonance form of ClO₃⁻.
