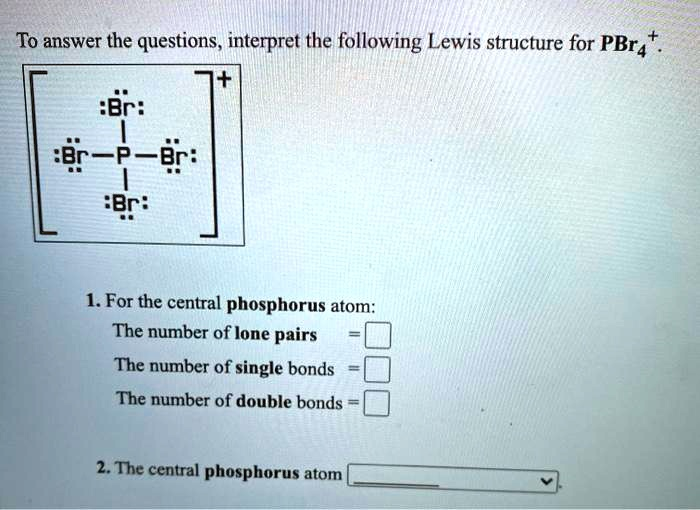
To answer the questions, interpret the following Lewis structure for PBr
. [Br :Br-P-Br: :Br:]$^+ 1. For the central phosphorus atom: The number of lone pairs = The number of single bonds = The number of double bonds = 2. The central phosphorus atom
The Correct Answer and Explanation is:
Based on the provided Lewis structure for PBr₄⁺, the central phosphorus atom is bonded to four bromine atoms, and each bond appears to be a single covalent bond. Here’s how we can answer the questions:
- For the central phosphorus atom: The number of lone pairs = 0 The number of single bonds = 4 The number of double bonds = 0
- The central phosphorus atom has an expanded octet with four bonding pairs and no lone pairs. It carries a formal positive charge in this cation.
Explanation:
To interpret the structure accurately, consider the electron count and bonding around phosphorus:
- Phosphorus is in Group 15 and typically has five valence electrons.
- In this PBr₄⁺ structure, it forms four single bonds, one with each bromine atom.
- Each Br atom contributes one electron to the bond, so phosphorus shares four of its electrons in covalent bonding.
- The molecule has a +1 charge, which means the phosphorus atom has one fewer electron than its neutral state, reducing its total from five to four valence electrons.
Thus, phosphorus uses all its valence electrons to form four sigma bonds and retains no nonbonding lone pairs.
Because there are four bonding pairs around phosphorus and no lone pairs, the electron geometry is tetrahedral. This geometry helps minimize repulsion between bonding electron pairs, aligning with the VSEPR theory.
The absence of double bonds is supported by the fact that each Br atom satisfies its octet with three lone pairs and one bonding pair. There is no need for pi bonding in this structure.
In summary, phosphorus in PBr₄⁺ forms four single bonds, has no lone pairs or double bonds, and the positive charge reflects the loss of one electron. The overall structure is stable due to proper octet fulfillment for all involved atoms.
