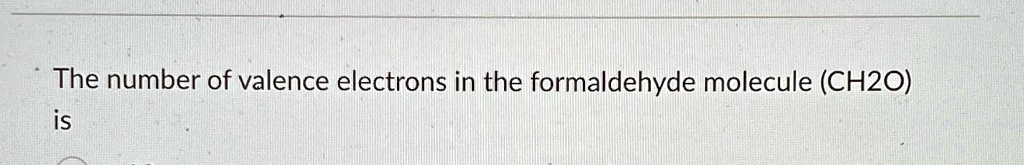
The number of valence electrons in the formaldehyde molecule ((CH₂O)
The Correct Answer and Explanation is:
The number of valence electrons in the formaldehyde molecule (CH₂O) is 12.
Explanation:
To find the number of valence electrons in a molecule, you sum the valence electrons of each atom in the molecule based on their positions in the periodic table.
Step 1: Count the atoms in CH₂O
- Carbon (C): 1 atom
- Hydrogen (H): 2 atoms
- Oxygen (O): 1 atom
Step 2: Find the number of valence electrons for each element
- Carbon is in group 14 → 4 valence electrons
- Hydrogen is in group 1 → 1 valence electron (per atom)
- Oxygen is in group 16 → 6 valence electrons
Step 3: Multiply by the number of each atom and sum
- Carbon: 1 × 4 = 4 electrons
- Hydrogen: 2 × 1 = 2 electrons
- Oxygen: 1 × 6 = 6 electrons
- Total: 4 + 2 + 6 = 12 valence electrons
Importance of Valence Electrons:
Valence electrons are crucial because they determine how atoms bond with each other to form molecules. In formaldehyde, the carbon atom forms a double bond with the oxygen atom and single bonds with each hydrogen atom. The distribution of these 12 valence electrons allows the molecule to achieve stable electron configurations for each atom based on the octet rule, except for hydrogen which only requires two electrons.
Understanding the total number of valence electrons helps in drawing the Lewis structure of a molecule. It ensures correct placement of bonding and non-bonding electron pairs, which in turn influences molecular shape, polarity, and reactivity. Formaldehyde is a simple but significant compound in organic chemistry, and recognizing its electron structure is key to understanding its chemical behavior.
