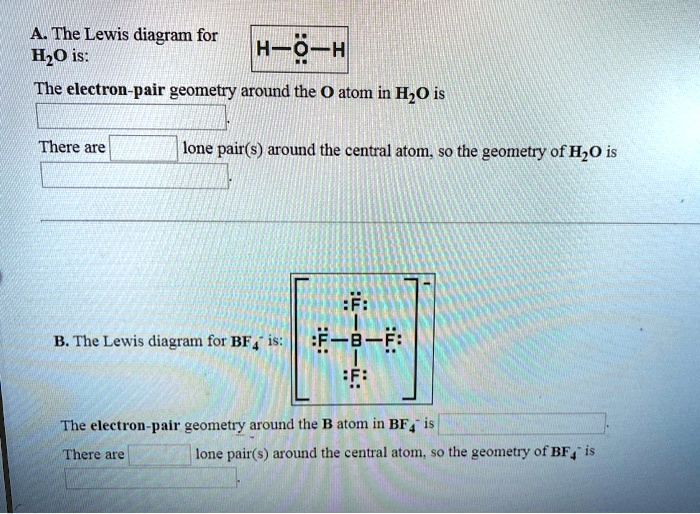
The Lewis diagram for H?O is: H-Ö-H The electron-pair geometry around the O atom in H?O is There are lone pair(s) around the central atom, so the geometry of H?O is :F: B. The Lewis diagram for BF?? is: [:F-B-F:]? :F: The electron-pair geometry around the B atom in BF?? is There are lone pair(s) around the central atom, so the geometry of BF?? is
The Correct Answer and Explanation is:
Correct Answers:
A. H₂O (Water)
- Electron-pair geometry: Tetrahedral
- Lone pairs: 2
- Molecular geometry: Bent
B. BF₄⁻ (Tetrafluoroborate ion)
- Electron-pair geometry: Tetrahedral
- Lone pairs: 0
- Molecular geometry: Tetrahedral
Explanation
In the water molecule (H₂O), oxygen is the central atom. Oxygen has six valence electrons and forms two covalent bonds with hydrogen atoms. This leaves two lone pairs on the oxygen atom. Altogether, there are four regions of electron density (two bonding pairs and two lone pairs). According to the VSEPR (Valence Shell Electron Pair Repulsion) theory, four electron regions arrange themselves in a tetrahedral electron-pair geometry to minimize repulsion. However, the two lone pairs repel the bonding pairs more strongly, compressing the bond angle between hydrogen atoms and creating a bent molecular geometry. Therefore, water has a tetrahedral electron-pair geometry but a bent molecular shape due to the lone pairs.
In the tetrafluoroborate ion (BF₄⁻), boron is the central atom. It is bonded to four fluorine atoms, each forming a single bond. The negative charge indicates the addition of one electron, making a total of eight valence electrons used in bonding. Boron forms four bonds and has no lone pairs. With four regions of bonding electron density and no lone pairs, the electron-pair geometry around boron is tetrahedral. Since there are no lone pairs to distort the geometry, the molecular shape is also tetrahedral. This is a classic example of symmetric electron distribution, resulting in equal bond angles of approximately 109.5 degrees.
In both molecules, the electron-pair geometry is tetrahedral due to the presence of four regions of electron density. The difference in their final molecular shapes arises from whether the central atom holds any lone pairs. The absence or presence of lone pairs affects the repulsion forces, and consequently, the overall shape of the molecule.
