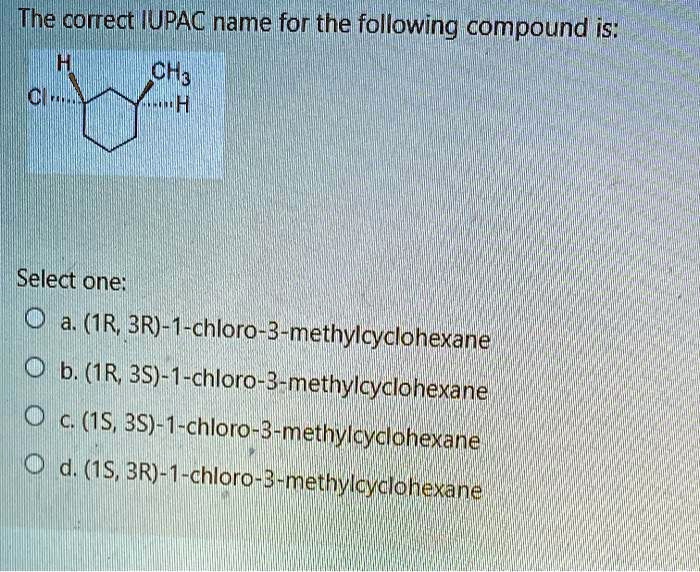
The correct IUPAC name for the following compound is: (IR; 3R)-1-chloro-3-methylcyclohexane.
The Correct Answer and Explanation is:
The correct answer is d. (1S, 3R)-1-chloro-3-methylcyclohexane.
Here is a step-by-step explanation for determining the correct IUPAC name:
1. Identify the Parent Chain and Numbering
The main structure is a six-membered carbon ring, which is named cyclohexane. There are two substituents on the ring: a chlorine atom (chloro group) and a methyl group (CH3).
According to IUPAC nomenclature rules, we must number the carbons in the ring to give the substituents the lowest possible numbers (locants). We also consider alphabetical order when there is a choice. “Chloro” comes before “methyl” alphabetically. Therefore, the carbon atom bonded to the chlorine atom is assigned position 1 (C1).
Next, we number the rest of the ring to give the methyl group the lowest possible number. Numbering clockwise from C1 places the methyl group at position 3. Numbering counter-clockwise would place it at position 5. Since 3 is lower than 5, we number the ring clockwise. This establishes the base name as 1-chloro-3-methylcyclohexane.
2. Determine the Stereochemistry (R/S Configuration)
The molecule has two chiral centers (carbon atoms attached to four different groups) at C1 and C3. We need to determine the absolute configuration (R or S) for each using the Cahn-Ingold-Prelog (CIP) priority rules.
Configuration at C1:
- Assign Priorities: The four groups attached to C1 are Cl, H, C2, and C6. Priorities are assigned based on atomic number.
- Cl (atomic number 17) is priority 1.
- H (atomic number 1) is priority 4.
- To distinguish between C2 and C6, we look at the atoms they are connected to. C2 leads to the methyl-substituted C3, while C6 leads to the unsubstituted C5. The path towards the substituent (C2) has higher priority. So, C2 is priority 2, and C6 is priority 3.
- Determine R/S: The priority sequence is Cl (1) > C2 (2) > C6 (3) > H (4). The lowest priority group (H) is on a wedge, pointing towards the viewer. Tracing the path from 1 to 2 to 3 is clockwise, which normally indicates R. However, because the lowest priority group points towards us, we must reverse the configuration. Thus, the configuration at C1 is S.
Configuration at C3:
- Assign Priorities: The four groups attached to C3 are CH3, H, C2, and C4.
- H is the lowest priority (4).
- We compare the three carbon groups. C2 leads to C1, which is bonded to a chlorine atom. C4 leads to unsubstituted carbons. The methyl carbon is only attached to hydrogens. The path toward the atom with the highest atomic number (Cl via C2) gets the highest priority. Therefore, C2 is priority 1.
- Comparing C4 and the methyl group, C4 is part of the ring and bonded to other carbons, giving it a higher priority than the terminal methyl group. So, C4 is priority 2, and CH3 is priority 3.
- Determine R/S: The priority sequence is C2 (1) > C4 (2) > CH3 (3) > H (4). The lowest priority group (H) is on a dash, pointing away from the viewer. Tracing the path from 1 to 2 to 3 is clockwise, which indicates an R configuration. Since the lowest priority group is pointing away, the assignment stands. The configuration at C3 is R.
3. Assemble the Full Name
By combining the stereochemical descriptors with the base name, we get the complete IUPAC name: (1S, 3R)-1-chloro-3-methylcyclohexane
