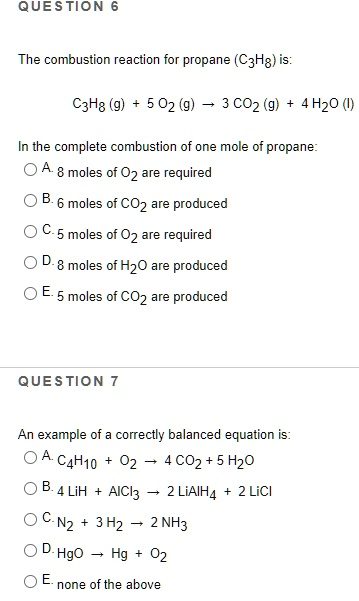
The combustion reaction for propane (C3H8) is:
The Correct Answer and Explanation is:
Correct Answers:
- Question 6: C. 5 moles of O₂ are required
- Question 7: C. N₂ + 3 H₂ → 2 NH₃
Explanation:
In Question 6, the combustion of propane (C₃H₈) is represented by the balanced equation: C₃H₈ + 5 O₂ → 3 CO₂ + 4 H₂O
This reaction describes the complete combustion of one mole of propane. By examining the stoichiometric coefficients in the equation, it is evident that one mole of propane reacts with 5 moles of oxygen gas (O₂) to produce 3 moles of carbon dioxide (CO₂) and 4 moles of water (H₂O). Therefore, the correct option is C, as it directly matches the balanced equation. It is important to note that complete combustion requires a sufficient supply of oxygen to fully oxidize carbon to CO₂ and hydrogen to H₂O. This distinguishes complete combustion from incomplete combustion, where products like CO or elemental carbon can form due to oxygen deficiency.
In Question 7, the goal is to identify a properly balanced chemical equation. Option C represents the well-known Haber process for synthesizing ammonia. The correct stoichiometry is: N₂ + 3 H₂ → 2 NH₃
Here, one molecule of nitrogen gas reacts with three molecules of hydrogen gas to produce two molecules of ammonia. The law of conservation of mass is satisfied because there are equal numbers of nitrogen and hydrogen atoms on both sides of the equation. This balanced equation not only aligns with theoretical predictions but also serves as a real-world industrial process in fertilizer production.
The other options in Question 7 either misrepresent the coefficients or do not balance correctly. For example, in option A, the number of oxygen atoms on each side does not match. Therefore, only option C satisfies the criteria for a balanced chemical reaction.
