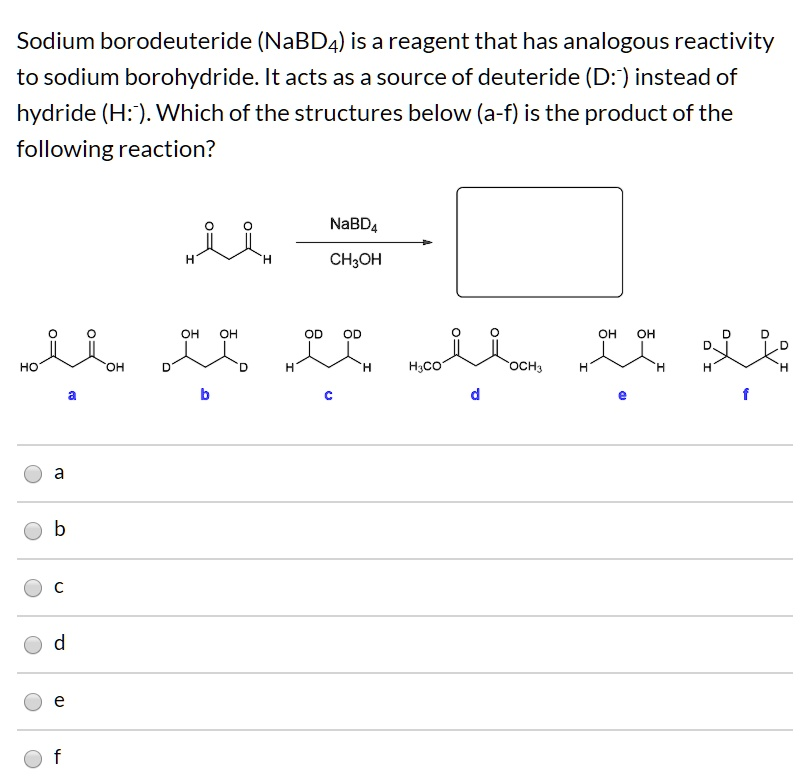
Sodium borodeuteride (NaBD4) is a reagent that has analogous reactivity to sodium borohydride. It acts as a source of deuteride (D-) instead of hydride (H-). Which of the structures below (a-f) is the product of the following reaction? NaBD4 + CH3OH OH OH OH HO- OH H2CO OCH3
The Correct Answer and Explanation is:
The correct answer is b.
Explanation:
The starting material shown in the reaction is 2,3-butanedione (also known as diacetyl), which is a diketone with the structure:
CopyEditCH3–CO–CO–CH3
In the reaction, this compound is treated with NaBD4 in methanol (CH3OH). Sodium borodeuteride behaves like sodium borohydride (NaBH4), but instead of donating hydride ions (H⁻), it donates deuteride ions (D⁻).
Reduction of Ketones:
NaBD4 reduces ketones to secondary alcohols by delivering a deuteride to the carbon of the carbonyl group (C=O), breaking the double bond and converting it to a hydroxyl group (-OH), while the carbon picks up a deuterium atom.
Because 2,3-butanedione has two ketone groups, each one will be reduced by NaBD4. So, both carbonyl groups are converted into hydroxyl groups, and each carbon that was originally part of a carbonyl group gains a deuterium atom.
Step-by-step transformation:
- Before Reaction: CH3–CO–CO–CH3 (a diketone)
- After Reaction with NaBD4: Each C=O becomes C–OD (from methanol as solvent, the OH forms, but the D comes from the NaBD4)
- The carbon atom of the C=O gets a D attached from the NaBD4, and the oxygen becomes an -OH group (protonated from the solvent).
So, the final product is:
CH3–C(OD)–C(OD)–CH3
But in practice, the –OD is rapidly protonated by methanol to form –OH, so the alcohol groups appear as –OH, and the D remains at the carbon.
Hence, the product is:
CH3–CH(OD)–CH(OD)–CH3
Which corresponds to structure b – a molecule with two hydroxyl groups and two deuterium atoms added to the carbons that were formerly carbonyls.
This is why b is the correct answer.
