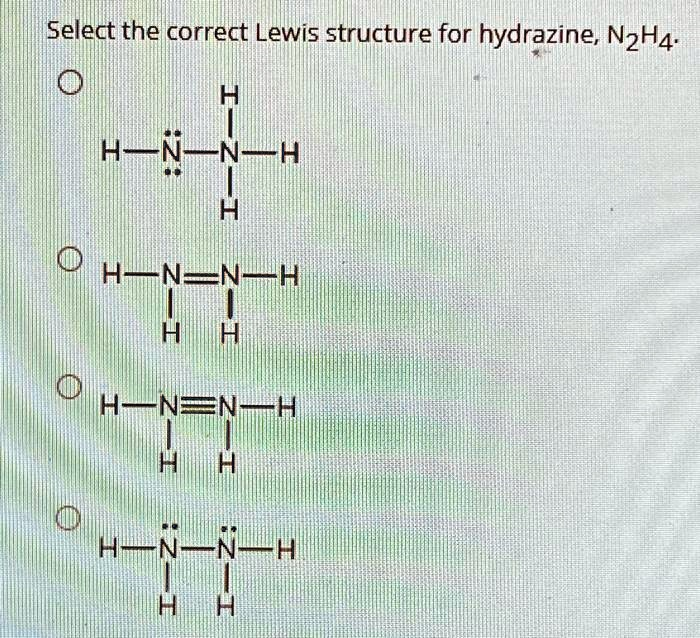
Select the correct Lewis structure for hydrazine, N2H4. H H-N-N-H H H H-N=N-H H H H-N?N-H H H H-N-N-H H H
The Correct Answer and Explanation is:
The correct Lewis structure for hydrazine (N₂H₄) is:
H — N — N — H | | H H
Each nitrogen atom in this structure is single-bonded to the other nitrogen and to two hydrogen atoms, and also has one lone pair of electrons. This structure satisfies the octet rule for each nitrogen atom and provides an accurate depiction of the bonding and geometry of hydrazine.
Hydrazine contains a total of 16 valence electrons. Each nitrogen contributes 5, and each hydrogen contributes 1. The goal in drawing the Lewis structure is to place these electrons so that each atom achieves a stable configuration.
Start with a single bond between the two nitrogen atoms. Each nitrogen then forms two additional bonds with hydrogen atoms to complete its bonding requirements. This gives each nitrogen three sigma bonds. Because nitrogen has 5 valence electrons, and it uses 3 in bonding, each atom retains one lone pair of electrons.
Placing a double bond between the nitrogen atoms would leave at least one nitrogen atom with too few hydrogen bonds or violate the octet rule by forcing it to accommodate more than 8 electrons. Likewise, a structure with a triple bond or with fewer hydrogen atoms violates the rules of typical valence bonding.
The correct structure also matches the known geometry of hydrazine. Its shape is similar to that of hydrogen peroxide (H₂O₂), with a non-planar, twisted N—N backbone due to lone pair repulsion. This confirms that a single bond between the nitrogens, with lone pairs on each, is consistent with both theoretical and experimental data.
Thus, the structure shown in the first option is correct.
