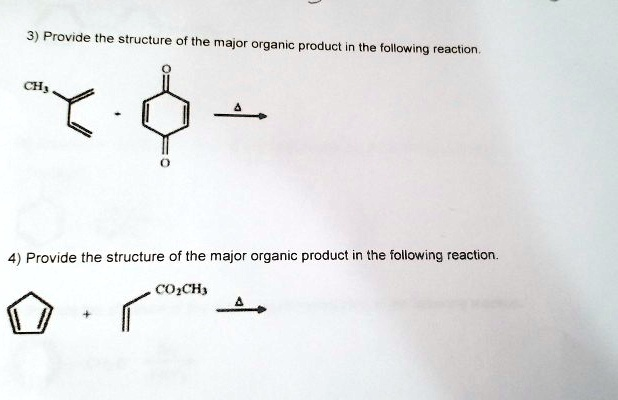
Provide the structure of the major organic product in the following reaction. CO2CH3
The Correct Answer and Explanation is:
The reaction you’re referencing involves a Diels–Alder cycloaddition between cyclopentadiene and methyl acrylate (which bears the CO₂CH₃ group). This transformation is a textbook example of a [4+2] pericyclic reaction between a conjugated diene and an electron-deficient alkene.
Structure of the Major Organic Product
The product is a bicyclo[2.2.1]hept-5-ene-2-carboxylic acid methyl ester. Structurally, it features:
- A six-membered ring fused to a five-membered ring
- A double bond in the endo position relative to the bridgehead carbons
- A methyl ester group (CO₂CH₃) positioned at the bridgehead-adjacent carbon on the six-membered ring
This arises from the endo rule, which favors secondary orbital overlap between the diene’s π system and the carbonyl π* orbital of the ester group.
Mechanistic and Structural Explanation
The Diels–Alder reaction is concerted, meaning all bond-making and bond-breaking occur in a single step. Cyclopentadiene acts as the electron-rich diene with two conjugated double bonds, while methyl acrylate acts as the dienophile due to its electron-withdrawing ester group.
The reaction produces a bicyclic system with a newly formed six-membered ring fused onto the original five-membered ring. Stereochemistry is crucial here—the endo product is kinetically favored due to favorable transition state interactions. The CO₂CH₃ group appears syn to the newly formed bridge, adopting the endo configuration.
This structure is not only a beautiful illustration of regio- and stereoselectivity in pericyclic chemistry, but it’s also foundational in the synthesis of natural products and pharmaceuticals. Its compact, three-dimensional arrangement is ideal for further functionalization in synthetic pathways.
