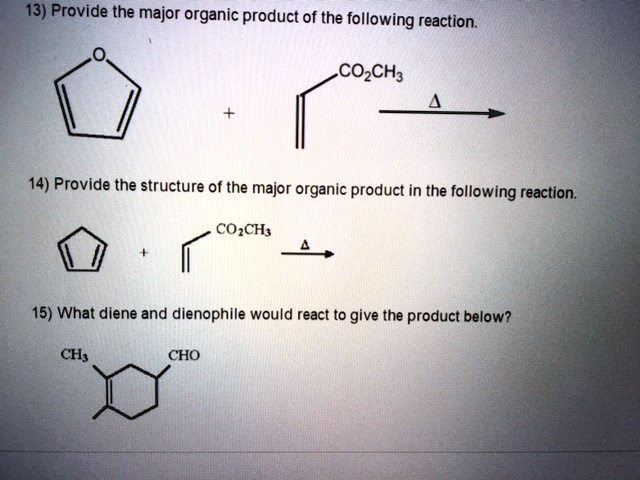
3) Provide the major organic product of the following reaction. CO
CH
? + 14) Provide the structure of the major organic product in the following reaction. CO
CH
- 15) What diene and dienophile would react to give the product below? CH
CHO
The Correct Answer and Explanation is:
Here are the answers and a detailed explanation for the three questions, all of which involve Diels–Alder reactions, a cornerstone of cycloaddition chemistry:
13) Major organic product of the reaction involving cyclopentadiene and methyl acrylate (CH₂=CH–COOCH₃): The reaction proceeds via a [4+2] cycloaddition. Cyclopentadiene acts as the diene, and methyl acrylate is the dienophile. The product is a bicyclic compound where the newly formed six-membered ring bears a methyl ester substituent at the bridgehead-adjacent carbon. The stereochemistry is endo due to secondary orbital overlap.
14) Major organic product from furan reacting with methyl acrylate (CH₂=CH–COOCH₃): Furan is an aromatic heterocycle that can serve as a diene under Diels–Alder conditions. With methyl acrylate as the dienophile, the reaction forms a 6-membered oxygen-containing ring fused to a 5-membered lactone-like system. This cycloadduct is less stable than traditional Diels–Alder products due to the potential loss of aromaticity but forms under mild conditions.
15) What diene and dienophile yield the shown product (4-methyl-3-cyclohexene-1-carbaldehyde)? The product suggests a 1,4-disubstituted cyclohexene with a methyl and aldehyde group. This points to:
- Diene: isoprene (2-methyl-1,3-butadiene)
- Dienophile: acrolein (CH₂=CH–CHO) The electron-deficient nature of the aldehyde-activated alkene favors cycloaddition.
Explanation The Diels–Alder reaction involves the [4+2] cycloaddition of a conjugated diene and a dienophile, forming a six-membered ring in one concerted step. It is stereospecific, regioselective, and generally thermally driven.
In question 13, cyclopentadiene is a highly reactive diene due to its s-cis conformation, while methyl acrylate offers an electron-withdrawing ester group that activates the alkene toward cycloaddition. The resulting adduct retains the bicyclic scaffold, making it synthetically useful in natural product synthesis.
Question 14 showcases furan, an aromatic heterocycle that can reversibly engage in Diels–Alder chemistry. Reaction with methyl acrylate yields an oxygen-containing heterocyclic product. Although the reaction disrupts aromaticity, it proceeds under mild conditions due to enhanced reactivity and solubility of the partners.
In question 15, the reverse analysis requires deconstructing the product into possible precursors. Isoprene, with its branched methyl group, aligns well with the methyl-substituted product, while acrolein contributes the aldehyde moiety. The endo product predominates due to stabilizing interactions in the transition state.
These three reactions highlight the versatility of the Diels–Alder reaction as a strategy for rapidly constructing complex molecular frameworks with functional diversity.
