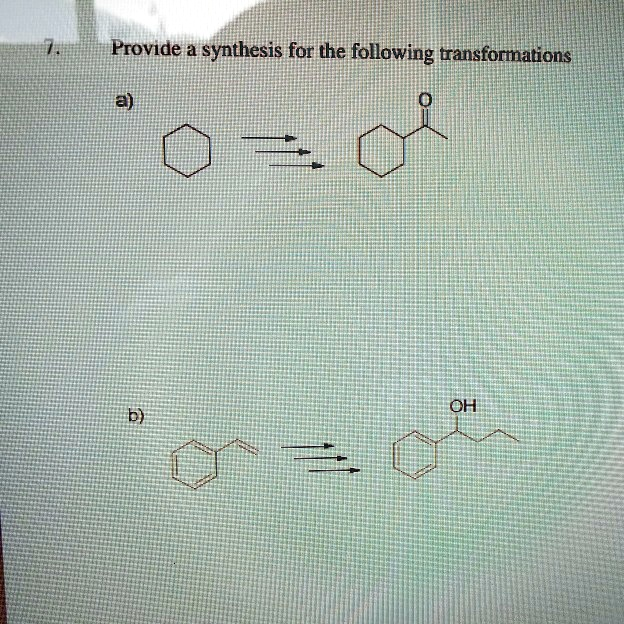
Provide a synthesis for the following transformations a) b) OH
The Correct Answer and Explanation is:
a) Cyclohexane → Cyclohexanone
Step 1: Oxidation Cyclohexane reacts with molecular oxygen in the presence of a cobalt catalyst to form cyclohexanol and cyclohexanone (mixture).
Step 2: Selective Oxidation Cyclohexanol can be oxidized using an oxidizing agent such as sodium hypochlorite (NaOCl) or PCC to yield cyclohexanone.
b) Styrene → 1-Phenyl-1-butanol
Step 1: Hydroboration-Oxidation Styrene undergoes hydroboration with BH₃ followed by oxidation with H₂O₂ in basic conditions to produce 2-phenylethanol.
Step 2: Conversion to Grignard Reagent 2-phenylethanol is converted to 2-phenylethyl bromide using PBr₃, then reacted with magnesium in dry ether to form the Grignard reagent (C₆H₅CH₂CH₂MgBr).
Step 3: Nucleophilic Addition This Grignard reagent reacts with propanal (CH₃CH₂CHO). After acidic workup, the final product is 1-phenyl-1-butanol.
Explanation
In transformation (a), the conversion of cyclohexane to cyclohexanone involves oxidation of an unreactive alkane. Direct oxidation using molecular oxygen in the presence of a cobalt catalyst yields both cyclohexanol and cyclohexanone. To selectively obtain cyclohexanone, cyclohexanol can be further oxidized. A common laboratory oxidizing agent like PCC or sodium hypochlorite allows this oxidation without over-oxidation to adipic acid.
In transformation (b), we begin with styrene, a vinylbenzene compound. Hydroboration-oxidation across the alkene introduces the hydroxyl group at the terminal carbon, following anti-Markovnikov selectivity, resulting in 2-phenylethanol. This intermediate is converted to a halide to enable Grignard reagent formation. Treatment with magnesium in anhydrous ether forms the nucleophilic organomagnesium compound.
The Grignard reagent now acts as a nucleophile and adds to an aldehyde carbonyl. Here, we use propanal to extend the carbon chain. The nucleophilic carbon from the Grignard attacks the electrophilic carbon of propanal. After the reaction is quenched with aqueous acid, a secondary alcohol, specifically 1-phenyl-1-butanol, is obtained. This sequence builds the carbon skeleton while maintaining the phenyl substituent on the terminal carbon, ensuring regioselectivity of the transformation.
These steps illustrate the strategic application of oxidation, hydroboration, and carbon–carbon bond formation using organometallic chemistry.
