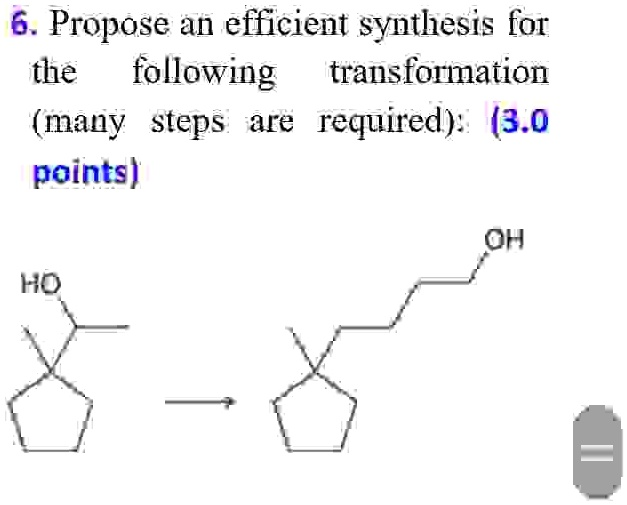
Propose an efficient synthesis for the following transformation (many steps are required): (3.0 points) 6.Propose an efficient synthesis for the following transformation many steps are required)3.0 points} OH HO
The Correct Answer and Explanation is:
To achieve the transformation of a substituted cyclopentane bearing an OH group into a regioisomer with the OH group on a different side chain, we can adopt a multi-step synthesis strategy. The objective is to reconfigure the side chain bearing the hydroxyl functionality through a series of strategic carbon–carbon bond formations and functional group interconversions. Here’s a plausible synthetic route:
Stepwise synthesis strategy
- Oxidation: Convert the original alcohol into a ketone using PCC (pyridinium chlorochromate).
- Wittig Reaction: React the ketone with a stabilized ylide to produce an exocyclic alkene.
- Ozonolysis: Cleave the double bond with ozone followed by reductive workup to generate an aldehyde.
- Grignard Reaction: Introduce a new side chain by reacting the aldehyde with a Grignard reagent, leading to a secondary alcohol.
- Protection: Protect the newly formed OH group using TBDMS-Cl in the presence of imidazole to allow selective chemistry elsewhere.
- Oxidation: Oxidize adjacent secondary carbons to ketones using reagents like Jones reagent.
- Baeyer–Villiger Oxidation: Transform the ketone into an ester, effectively shifting the side chain position through rearrangement.
- Reduction: Reduce the ester into a primary alcohol using LiAlH4.
- Deprotection: Remove the protecting group from the original OH with TBAF.
- Purification: Use column chromatography to isolate the regioisomer.
Explanation This synthesis showcases the elegance of retrosynthetic logic. By converting the initial alcohol into a ketone, we enable nucleophilic additions that extend or shift the carbon framework. A Wittig reaction serves to reorient the molecular skeleton, while ozonolysis and Grignard addition strategically change connectivity. Functional group protection ensures orthogonality so that only the desired sites react. Baeyer–Villiger oxidation is employed to relocate the side chain by converting a ketone into an ester, which upon reduction yields the alcohol in the required position. This approach minimizes carbon loss and maximizes functional group economy while preserving the ring framework. It also exemplifies how oxidation–reduction and rearrangement chemistry can be choreographed to reshape molecular architecture effectively.
