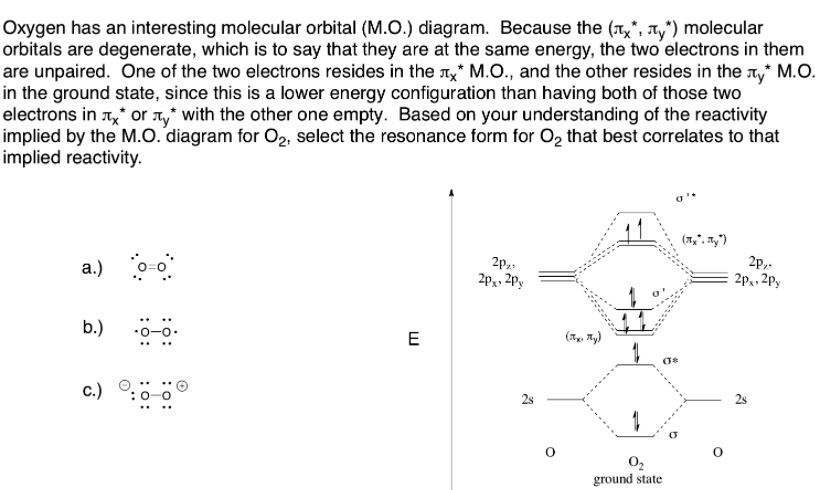
Oxygen has an interesting molecular orbital (M.O.) diagram: Because the (Tx Ty’) molecular orbitals are degenerate, which is to say that they are at the same energy, the two electrons in them are unpaired. One of the two electrons resides in the Ix* M.O., and the other resides in the Ty* M.O. in the ground state, since this is a lower energy configuration than having both of those two electrons in Tx or Ty’ with the other one empty. Based on your understanding of the reactivity implied by the M.O. diagram for O2, select the resonance form for O2 that best correlates to that implied reactivity: a: 2p^4. 11,4, ZPz 2, 20 ground state
The Correct Answer and Explanation is:
The correct resonance form for O₂ that best correlates with the molecular orbital (M.O.) diagram and its implied reactivity is none of the classical Lewis structures. Instead, the most accurate depiction involves a diradical resonance form showing two unpaired electrons on adjacent oxygen atoms, consistent with the electronic configuration predicted by M.O. theory.
From the M.O. diagram of O₂, we know the ground state configuration is:
σ1s², σ1s², σ2s², σ2s², (π2pₓ)², (π2p_y)², (π2pₓ)¹, (π2p_y)¹
This places a total of 12 valence electrons in bonding and antibonding orbitals. Importantly, the presence of two unpaired electrons in degenerate antibonding π* orbitals results in a bond order of:
(8 bonding electrons − 4 antibonding electrons) ÷ 2 = 2
Therefore, O₂ has a double bond overall, but the existence of unpaired electrons implies paramagnetism, which cannot be depicted accurately using traditional Lewis structures. Standard resonance forms such as O=O or O⁻–O⁺ fail to capture this feature because they predict all electrons are paired, which contradicts experimental evidence such as the attraction of O₂ to a magnetic field.
The best representation must therefore account for both a bond order of 2 and the two unpaired electrons. A conceptual resonance form can show an O=O double bond with one unpaired electron on each oxygen atom. While not a formal Lewis structure, this depiction is aligned with the molecular orbital model and observed physical properties.
This diradical nature explains O₂’s unique reactivity in combustion and oxidation reactions. Unlike typical singlet molecules, triplet oxygen reacts slowly with many substances because its unpaired electrons require spin pairing with reactants — a process often facilitated by catalysts or UV activation.
