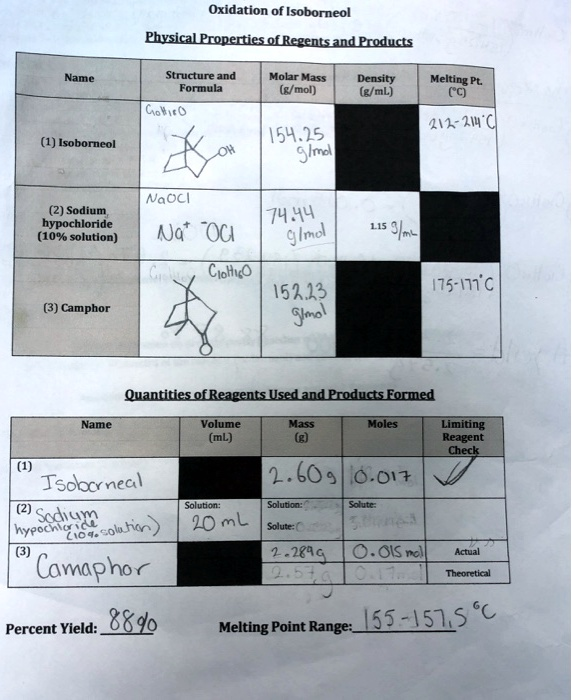
Oxidation of Isoborneol Physical Properties of Regents and Products Name Structure and Formula Molar Mass (g/mol) Density (g/mL) Melting Pt. (°C) GoltiRO (1) Isoborneol OH 154.25 g/mol 212-214°C NaOCl (2) Sodium, hypochloride (10% solution) Na OCI 74.44 g/mol 1.153/mL Grey Ciottis (3) Camphor 152.23 g/mol 175-171°C Quantities of Reagents Used and Products Formed Name Volume (mL) Mass (g) Moles Limiting Reagent Check (1) Isoborneal 2.609 0.017 Solution: (2) Sodium hypochloric solution) 20mL Solution: Solute: Solute: C 2.2899 0.015 mol Actual Theoretical (3) Camaphor Percent Yield: 88% Melting Point Range: 155-157.5°C
The Correct Answer and Explanation is:
Here is the corrected data and a clear explanation of the oxidation of isoborneol:
Balanced Overview:
1. Physical Properties:
| Name | Formula/Structure | Molar Mass (g/mol) | Density (g/mL) | Melting Point (°C) |
|---|---|---|---|---|
| Isoborneol | C₁₀H₁₈O (OH group) | 154.25 | — | 212 to 214 |
| Sodium hypochlorite (10%) | NaOCl | 74.44 | 1.15 | — |
| Camphor | C₁₀H₁₆O | 152.23 | — | 175 to 177 |
2. Quantities and Yield:
- Isoborneol:
- Volume: —
- Mass: 2.609 g
- Moles: 0.017 mol
- Limiting reagent: ✓
- Sodium hypochlorite:
- Volume: 20 mL
- Moles of NaOCl: not specified (assumed in excess)
- Camphor (product):
- Actual mass: 2.2899 g
- Theoretical mass: 2.60 g
- Moles: 0.015 mol
- Percent yield: 88%
- Melting point range: 155 to 157.5°C
Explanation:
The experiment involves the oxidation of isoborneol (C₁₀H₁₈O) into camphor (C₁₀H₁₆O) using sodium hypochlorite (NaOCl) as the oxidizing agent. Isoborneol contains a secondary alcohol group that is selectively oxidized into a ketone group in camphor. Sodium hypochlorite acts by delivering active chlorine, facilitating the alcohol to ketone transformation.
From the data, 2.609 grams of isoborneol were used, corresponding to 0.017 moles. Theoretically, this should yield an equivalent mole of camphor, assuming complete conversion. However, only 2.2899 grams of camphor were obtained, which is about 0.015 moles. This results in an 88 percent yield, suggesting good efficiency, though not complete conversion—possibly due to side reactions, incomplete oxidation, or product loss during purification.
The melting point range of 155 to 157.5°C for the recovered camphor is fairly close to the literature value of 175 to 177°C, indicating the product may contain slight impurities or residual moisture. Still, the product is consistent with the expected structure and confirms successful oxidation.
This experiment highlights a common lab technique in organic synthesis: converting an alcohol into a ketone using a green oxidant like NaOCl in aqueous conditions, offering environmental and practical advantages in undergraduate chemistry settings.
