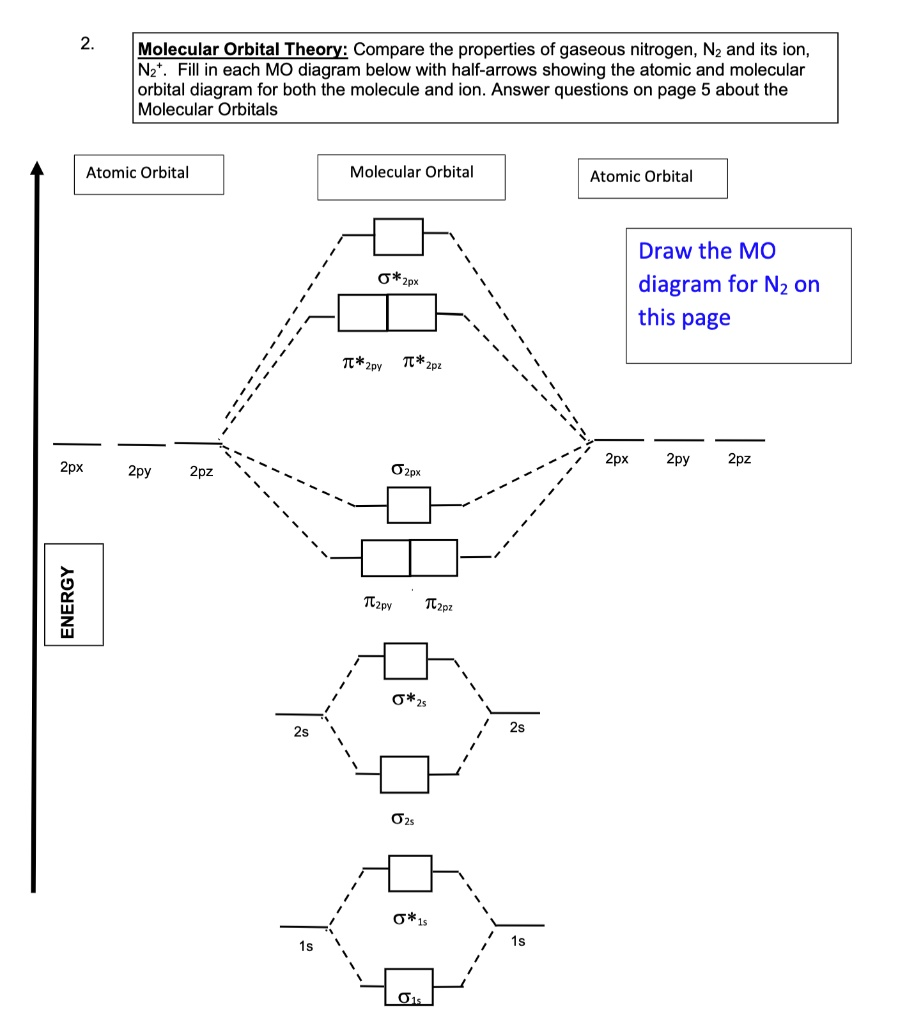
. Molecular Orbital Theory: Compare the properties of gaseous nitrogen, N2 and its ion, N2+. Fill in each MO diagram below with half-arrows showing the atomic and molecular orbital diagram for both the molecule and ion. Answer questions on page 5 about the Molecular Orbitals Atomic Orbital Molecular Orbital Atomic Orbital ?2px ?2py ?2pz Draw the MO diagram for N2 on this page 2px 2py 2pz 2px 2py 2pz ?2px ENERGY 2s 1s ?2py ?2pz ?2s ?2s ?*1s ?1s 2s 1
The Correct Answer and Explanation is:
To construct the molecular orbital (MO) diagrams for N₂ and N₂⁺, we must first consider the atomic number of nitrogen, which is 7. Each nitrogen atom contributes 7 electrons, so N₂ has a total of 14 electrons, and N₂⁺ has 13.
Molecular Orbital Diagram (simplified ordering for N₂ and lighter diatomic elements):
Molecular orbital energy ordering:
- σ(1s), σ*(1s)
- σ(2s), σ*(2s)
- π(2pₓ) = π(2pᵧ), σ(2p_z),
- π(2pₓ) = π(2pᵧ), σ*(2p_z)
Electron Configuration:
- N₂ (14 electrons): σ(1s)², σ(1s)², σ(2s)², σ(2s)², π(2pₓ)², π(2pᵧ)², σ(2p_z)² Bond order = ½[(8 bonding) – (2 antibonding)] = 3
- N₂⁺ (13 electrons): Same as N₂ but one electron fewer, so one from π(2pₓ) or π(2pᵧ) is removed Bond order = ½[(7 bonding) – (2 antibonding)] = 2.5
Explanation:
In molecular orbital theory, atomic orbitals combine to form bonding and antibonding orbitals. The number of electrons in these orbitals determines the bond order and magnetic properties. N₂ has a bond order of 3, indicating a very strong triple bond and high stability. It is diamagnetic because all electrons are paired.
N₂⁺ has one fewer electron, which is typically removed from the degenerate π(2pₓ) or π(2pᵧ) orbital. This reduces the bond order to 2.5 and introduces one unpaired electron, making it paramagnetic.
This change impacts both chemical reactivity and physical behavior. N₂⁺ is less stable and more reactive than neutral N₂. The MO diagram illustrates this by showing one less electron in the bonding region and an unpaired spin.
Understanding these differences helps explain phenomena like reactivity in ionized atmospheres or the behavior of molecular nitrogen in high-energy environments such as plasmas or flames.
