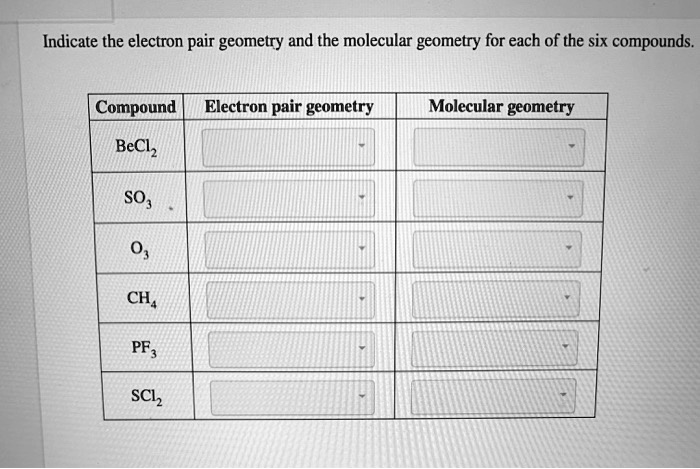
for each of the six compounds. Compound Electron pair geometry Molecular geometry BeCl2 SO2 PF3 CH4 SCl2
Indicate the electron pair geometry and the molecular geometry for each of the six compounds. Compound Electron pair geometry Molecular geometry BeCl2 SO2 PF3 CH4 SCl2
The Correct Answer and Explanation is:
Here’s the completed table followed by a detailed explanation:
| Compound | Electron Pair Geometry | Molecular Geometry |
|---|---|---|
| BeCl₂ | Linear | Linear |
| SO₂ | Trigonal planar | Bent |
| PF₃ | Tetrahedral | Trigonal pyramidal |
| CH₄ | Tetrahedral | Tetrahedral |
| SCl₂ | Tetrahedral | Bent |
Explanation:
To determine the electron pair and molecular geometries, we use VSEPR (Valence Shell Electron Pair Repulsion) theory, which considers both bonding and lone pairs around the central atom.
BeCl₂: Beryllium has two bonding pairs and no lone pairs, leading to a linear arrangement to minimize repulsion. Both electron pair and molecular geometry are linear.
SO₂: Sulfur is surrounded by two double bonds to oxygen and one lone pair. The presence of three electron regions gives it a trigonal planar electron geometry. However, the lone pair compresses the shape into a bent molecular geometry.
PF₃: Phosphorus forms three single bonds with fluorine and has one lone pair. Four electron regions yield a tetrahedral electron pair geometry. The lone pair causes a trigonal pyramidal molecular geometry.
CH₄: Carbon makes four single bonds to hydrogen with no lone pairs, resulting in both electron pair and molecular geometries being tetrahedral.
SCl₂: Sulfur is bonded to two chlorine atoms and has two lone pairs. Four electron regions give a tetrahedral electron geometry. The two lone pairs repel more strongly, causing the molecule to adopt a bent molecular geometry.
Understanding these shapes is vital for predicting molecular polarity, reactivity, and physical properties. Lone pairs influence the molecular shape even though they are not visible in the geometry itself. The spatial arrangement of atoms, governed by these geometries, plays a critical role in chemical behavior and intermolecular interactions.
