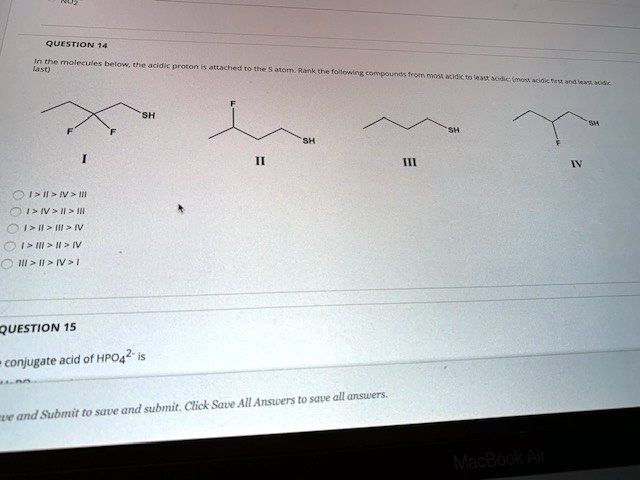
In the molecules below, the acidic proton is attached to the S atom. Rank the following compounds from most acidic to least acidic (most acidic first and least acidic last) SH F F I III IV > II > III IIIIII IV I>III > II > IV IIIII>IV>1 SH II 111 QUESTION 15 conjugate acid of HPO4
is ve and Submit to save and submit. Click Save All Answers to save all answers. SH MacBook Air IV SH
The Correct Answer and Explanation is:
To determine the acidity of the thiol compounds (R–SH) shown in the image, we must consider how the structure of each molecule affects the stability of the conjugate base (R–S⁻) after deprotonation. A more stable conjugate base corresponds to a more acidic compound. The major factors influencing acidity here are inductive effects, particularly from electronegative atoms like fluorine that withdraw electron density through sigma bonds. This stabilizes the negative charge on the sulfur atom after the proton is lost.
Structure Analysis:
- Compound I: Contains two fluorine atoms on the carbon adjacent to the SH group. These strong electron-withdrawing groups stabilize the conjugate base significantly through inductive effects.
- Compound II: Contains one fluorine atom on the β-carbon (one carbon away from the SH). While it has some electron-withdrawing effect, it’s weaker due to the increased distance from the sulfur atom.
- Compound III: A simple straight-chain thiol (butanethiol) with no electronegative substituents. Therefore, its conjugate base is not stabilized by inductive effects.
- Compound IV: Has one fluorine atom on the γ-carbon (two carbons away from the SH group), which offers minimal inductive stabilization due to the distance.
Ranking from Most Acidic to Least Acidic:
- Compound I – Strongest inductive effect due to two fluorine atoms on the adjacent carbon.
- Compound II – One fluorine on a nearby carbon gives moderate inductive stabilization.
- Compound IV – One fluorine but farther away, so the inductive effect is weaker.
- Compound III – No electron-withdrawing groups; least stabilized conjugate base.
Correct Answer:
I > II > IV > III
This corresponds to the last option in the multiple-choice answers.
Explanation Summary
Acidity in organic molecules like thiols is largely determined by the stability of their conjugate bases. When a thiol (R–SH) loses a proton (H⁺), it forms a thiolate anion (R–S⁻). The more stable this anion is, the more acidic the original thiol compound will be. In these examples, electronegative fluorine atoms play a crucial role. They stabilize the negative charge on the sulfur atom through inductive effects by pulling electron density away from the thiolate anion.
Compound I has the highest acidity because it contains two fluorine atoms directly adjacent to the sulfur. These fluorines exert a strong inductive effect, greatly stabilizing the conjugate base. Compound II has one fluorine on the β-carbon, which still provides some stabilization, though less than compound I. Compound IV has a fluorine on the γ-carbon, farther from the sulfur, which means the inductive effect is weaker. Finally, compound III has no electronegative substituents, so the thiolate formed upon deprotonation is not stabilized, making it the least acidic.
This trend illustrates how the presence and position of electronegative atoms influence the acidity of thiols. The closer and more numerous the electron-withdrawing groups are to the acidic proton, the more acidic the molecule becomes due to better stabilization of the conjugate base. Therefore, the correct order from most to least acidic is I > II > IV > III.
