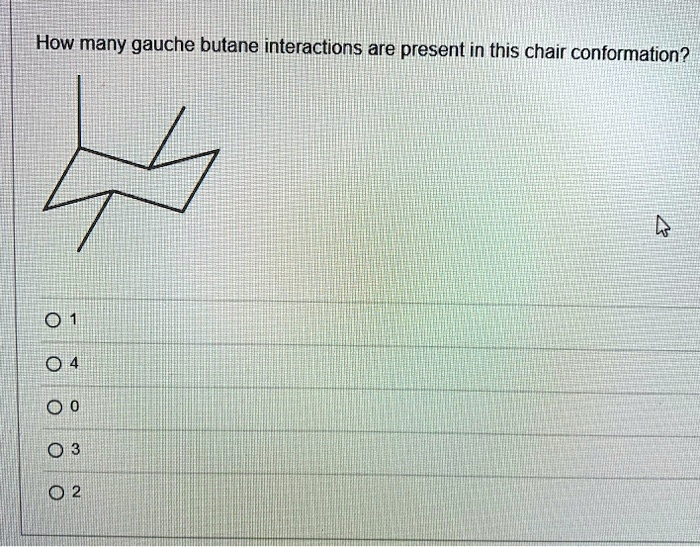
How many gauche butane interactions are present in this chair conformation? 01 04 00 03 02
The Correct Answer and Explanation is:
The correct answer is 4.
Here is a step-by-step explanation:
A gauche butane interaction is a type of steric strain that occurs when two methyl groups (or other non-hydrogen groups) in a butane-like fragment are positioned with a 60° dihedral angle. In cyclohexane chair conformations, these interactions are a key factor in determining the stability of different conformers. We count these interactions to estimate the overall steric strain introduced by substituents.
- Identify the Substituents and Their Positions:
First, let’s analyze the provided chair conformation. It is a substituted cyclohexane with three methyl groups.- One methyl group is in an axial position (pointing straight up).
- A second methyl group is also in an axial position (pointing straight down).
- The third methyl group is in an equatorial position (pointing out to the side, and slightly down).
- Apply the Rules for Counting Gauche Butane Interactions:
The standard method for counting these interactions in a substituted cyclohexane is as follows:- Axial Substituents: Each axial substituent (like a methyl group) creates two gauche butane interactions with the carbon-carbon bonds of the ring. This is because the axial substituent is gauche to the ring bonds located two carbons away (at the C-3 and C-5 positions relative to the substituent).
- Equatorial Substituents: An equatorial substituent is in an anti conformation relative to these same ring bonds. An anti conformation has a 180° dihedral angle and introduces no steric strain. Therefore, an equatorial substituent contributes zero gauche butane interactions with the ring.
- Calculate the Total Interactions for This Molecule:
Using the rules above, we can count the interactions for each substituent in the given molecule:- First axial methyl group: Contributes 2 gauche butane interactions.
- Second axial methyl group: Contributes 2 gauche butane interactions.
- Equatorial methyl group: Contributes 0 gauche butane interactions.
Total = 2 + 2 + 0 = 4
Therefore, there are a total of 4 gauche butane interactions present in this chair conformation. It is important to note that the very severe steric clash between the two axial methyl groups (a 1,3-diaxial interaction) is a different and more significant type of strain, not typically counted under the “gauche butane” label in this context.
