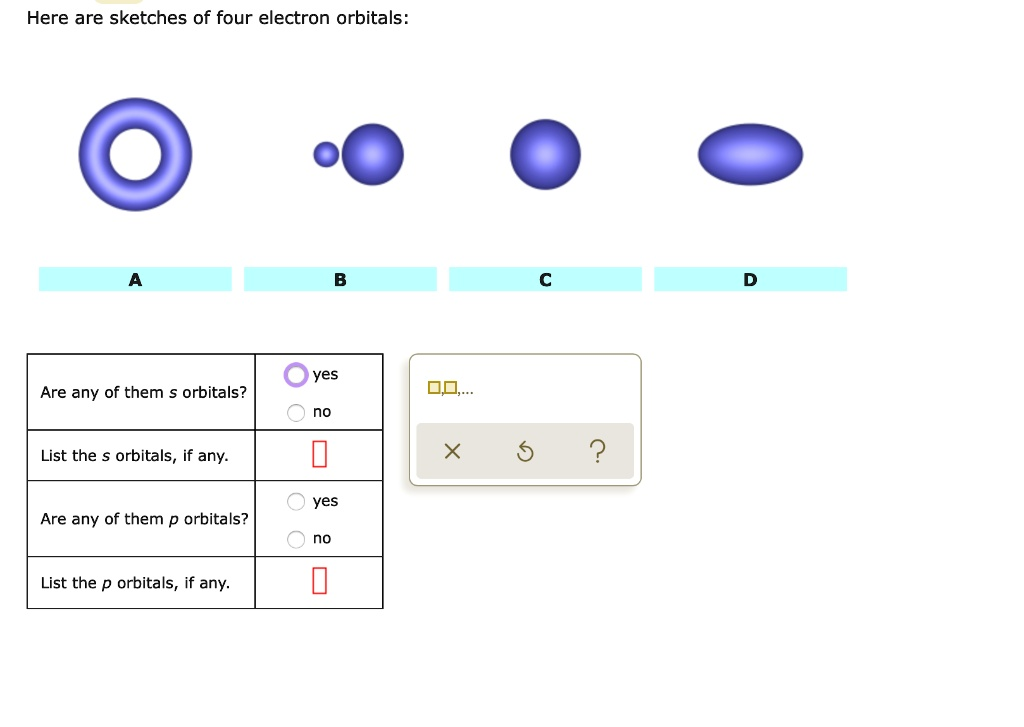
Here are sketches of four electron orbitals: yes Are any of them $ orbitals? List the orbitals, if any: 2 yes Are any of them p orbitals? List the orbitals if any_
The Correct Answer and Explanation is:
Correct answers: Are any of them s orbitals? Yes List the s orbitals: B, C Are any of them p orbitals? Yes List the p orbitals: D
Explanation: Electron orbitals represent regions around an atom’s nucleus where electrons are most likely found. These orbitals differ in shape and energy levels, which are crucial for understanding atomic structure and bonding behavior.
The s orbitals are always spherical in shape. This symmetry allows them to be non-directional and equally probable in all directions from the nucleus. In your sketches, orbitals B and C match this description. Orbital B displays two small spheres and likely represents an s orbital in a nodal state or higher energy level. Orbital C is a single sphere, which aligns with the typical representation of a 1s or 2s orbital.
The p orbitals have a characteristic dumbbell shape. These orbitals are directional, existing along specific axes (px, py, or pz). In the diagram, orbital D shows an elongated, oval shape that corresponds with this structure and suggests the presence of a p orbital.
Orbital A, however, resembles a ring or donut-like shape. This is uncharacteristic of both s and p orbitals and instead resembles a d orbital, which can appear in cloverleaf or toroidal configurations. D orbitals typically become relevant in atoms with electrons in the third energy level or higher.
Understanding orbital geometry helps explain not only electron configurations but also chemical bonding patterns and molecular shapes. It underpins theories like VSEPR and hybridization. In short, the shape of each orbital governs the types of bonds an atom can form and how it interacts within a molecule. By recognizing these orbitals visually, you gain a sharper intuition for electronic structure and reactivity. This insight is foundational to both inorganic and organic chemistry.
