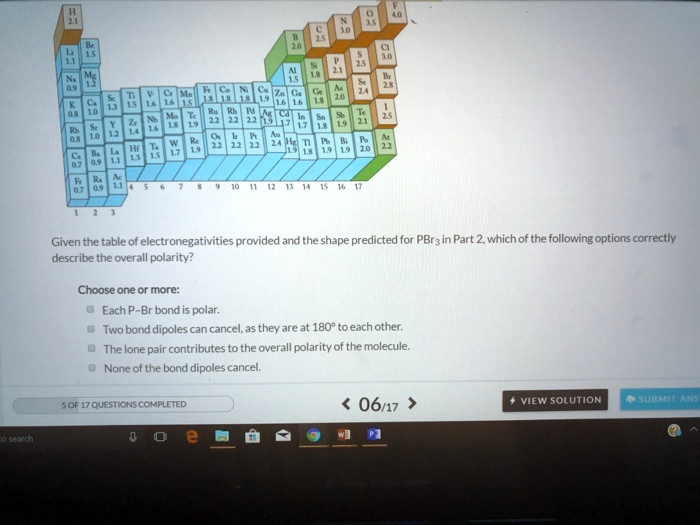
for PBr5 in Part 2, which of the following options correctly describe the overall polarity? Choose one or more: Each Br bond is polar. Two bond dipoles can cancel, as they are at 180° to each other. The lone pair contributes to the overall polarity of the molecule. None of the bond dipoles cancel. Gor17CUSTIONS COMPLETED 06/17 View Solution SUBMLAN Aenfch
Given the table of electronegativities provided and the shape predicted for PBr5 in Part 2, which of the following options correctly describe the overall polarity? Choose one or more: Each Br bond is polar. Two bond dipoles can cancel, as they are at 180° to each other. The lone pair contributes to the overall polarity of the molecule. None of the bond dipoles cancel. Gor17CUSTIONS COMPLETED 06/17 View Solution SUBMLAN Aenfch
The Correct Answer and Explanation is:
To determine the correct options about the polarity of PBr₅ (phosphorus pentabromide), we need to evaluate both its molecular shape and bond polarity, using the electronegativity table provided.
Step 1: Electronegativity Difference
From the chart:
- Electronegativity of P (Phosphorus) = 2.1
- Electronegativity of Br (Bromine) = 2.8
The difference = 2.8 – 2.1 = 0.7, which indicates polar covalent bonds.
So, each P–Br bond is polar.
Step 2: Molecular Geometry of PBr₅
PBr₅ has:
- 5 bonding pairs
- 0 lone pairs on phosphorus
This gives it a trigonal bipyramidal shape:
- Three Br atoms in the equatorial positions (120° apart)
- Two Br atoms in the axial positions (180° apart from each other and 90° from equatorial atoms)
Step 3: Polarity of the Molecule
Even though the P–Br bonds are polar, the molecule’s symmetrical trigonal bipyramidal shape allows the dipole moments to cancel out. The vector sum of the dipoles results in a nonpolar molecule.
Also:
- There is no lone pair on the central atom, so no lone-pair-induced asymmetry.
- The equatorial and axial dipoles are balanced and distributed symmetrically.
Correct Options:
✅ Each P–Br bond is polar
❌ Two bond dipoles can cancel, as they are at 180° to each other → Partially true for axial bonds, but oversimplified
❌ The lone pair contributes to the overall polarity of the molecule → Incorrect because there is no lone pair
❌ None of the bond dipoles cancel → Incorrect, they do cancel due to symmetry
Final Answer:
- ✅ Each P–Br bond is polar
