(36 pts) Fill in the boxes and blanks for SiS
Total VE Steric Number Geometry Shape Bond Angle Lewis Structure 3-D Structure FO
Total VE Steric Number Geometry Shape Bond Angle Lewis Structure SF
Total V
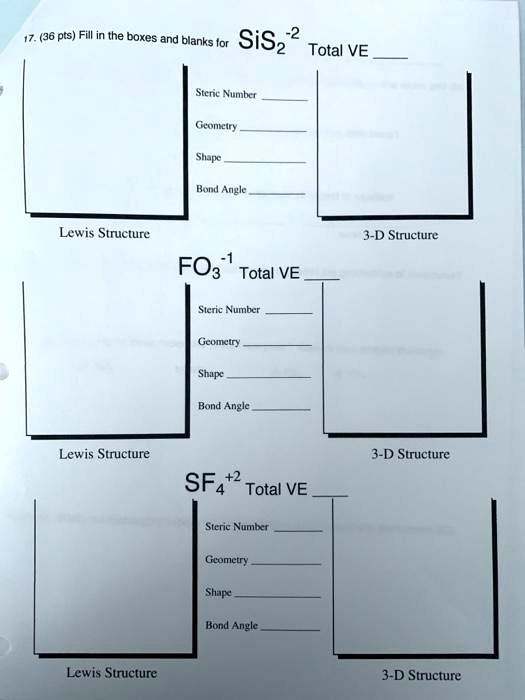
E Steric Number Geometry Shape Bond Angle Lewis Structure 3-D Structure 3-D Structure
The Correct Answer and Explanation is:
Let’s analyze and fill in the chart for each of the three species: SiS₂²⁻, FO₃⁻, and SF₄²⁺.
1. SiS₂²⁻
Total Valence Electrons (VE):
Si = 4, S = 6 × 2 = 12, charge = +2 ⇒ 4 + 12 + 2 = 18 VE
Steric Number: 2 (2 bonding domains, 0 lone pairs)
Geometry: Linear
Shape: Linear
Bond Angle: 180°
Lewis Structure:
pgsqlCopyEditS = Si = S (with each S having 3 lone pairs, double bonds to Si)
3D Structure: Straight line: S—Si—S
2. FO₃⁻
Total Valence Electrons (VE):
F = 7, O = 6 × 3 = 18, charge = +1 ⇒ 7 + 18 + 1 = 26 VE
Steric Number: 4 (3 bonding pairs, 1 lone pair)
Geometry: Tetrahedral
Shape: Trigonal pyramidal
Bond Angle: ~107°
Lewis Structure:
Central F with three single bonds to O (each O has 3 lone pairs), F has one lone pair
3D Structure: A trigonal pyramid with O atoms at the base and F at the top
3. SF₄²⁺
Total Valence Electrons (VE):
S = 6, F = 7 × 4 = 28, charge = −2 ⇒ 6 + 28 − 2 = 32 VE
(However, since the ion is SF₄²⁺, we subtract 2 electrons: 34 − 2 = 32 VE)
Steric Number: 4 (4 bonding pairs, 0 lone pairs)
Geometry: Tetrahedral
Shape: Tetrahedral
Bond Angle: ~109.5°
Lewis Structure:
Central S with four single bonds to F atoms, no lone pairs on S, each F has 3 lone pairs
3D Structure: Tetrahedral with S in the center and F atoms at the corners
Summary Table
| Molecule | Total VE | Steric No. | Geometry | Shape | Bond Angle |
|---|---|---|---|---|---|
| SiS₂²⁻ | 18 | 2 | Linear | Linear | 180° |
| FO₃⁻ | 26 | 4 | Tetrahedral | Trigonal Pyramidal | ~107° |
| SF₄²⁺ | 32 | 4 | Tetrahedral | Tetrahedral | ~109.5° |
These answers are based on standard valence-shell electron-pair repulsion (VSEPR) theory. This theory helps predict the molecular shape by considering repulsions between electron domains (bonding and lone pairs). A lower number of lone pairs usually leads to more symmetrical and regular shapes.
