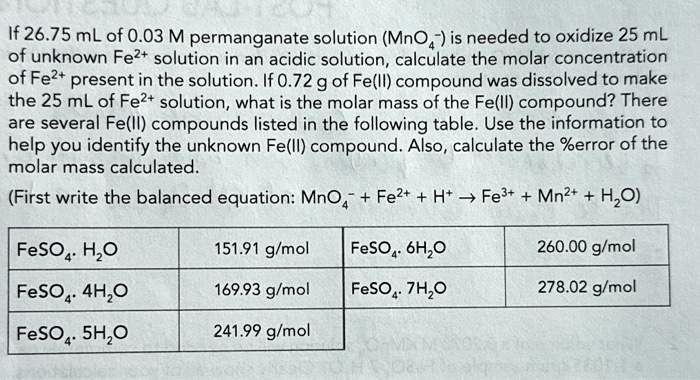
f 26.75 mL of 0.03 M permanganate solution (MnO4−) is needed to oxidize 25 mL of unknown Fe2+ solution in an acidic solution
The Correct Answer and Explanation is:
To solve the problem, we need to determine two things:
- The molar concentration of Fe²⁺ in the unknown solution.
- The molar mass of the Fe(II) compound used to prepare the solution.
Step 1: Write and balance the redox reaction
In acidic conditions:
MnO4−+5Fe2++8H+→Mn2++5Fe3++4H2O\text{MnO}_4^- + 5\text{Fe}^{2+} + 8\text{H}^+ \rightarrow \text{Mn}^{2+} + 5\text{Fe}^{3+} + 4\text{H}_2\text{O}
This tells us that 1 mole of permanganate reacts with 5 moles of Fe²⁺.
Step 2: Moles of MnO₄⁻ used
Given volume = 26.75 mL = 0.02675 L Given concentration = 0.03 mol/L
Moles MnO4−=0.03×0.02675=8.025×10−4 mol\text{Moles MnO}_4^- = 0.03 \times 0.02675 = 8.025 \times 10^{-4} \text{ mol}
Step 3: Moles of Fe²⁺ in 25 mL solution
From the balanced equation,
Moles Fe2+=5×Moles MnO4−=5×8.025×10−4=0.0040125 mol\text{Moles Fe}^{2+} = 5 \times \text{Moles MnO}_4^- = 5 \times 8.025 \times 10^{-4} = 0.0040125 \text{ mol}
Now calculate molar concentration of Fe²⁺ in 25 mL = 0.025 L:
Concentration of Fe2+=0.00401250.025=0.1605 mol/L\text{Concentration of Fe}^{2+} = \frac{0.0040125}{0.025} = 0.1605 \text{ mol/L}
Step 4: Determine molar mass
Given mass of Fe(II) compound = 0.72 g
Molar mass=0.72 g0.0040125 mol≈179.4 g/mol\text{Molar mass} = \frac{0.72 \text{ g}}{0.0040125 \text{ mol}} \approx 179.4 \text{ g/mol}
Step 5: Identify compound and calculate percent error
From the table, the closest match is FeSO₄·4H₂O (169.93 g/mol).
Percent error=∣179.4−169.93∣169.93×100≈5.57%\text{Percent error} = \frac{|179.4 – 169.93|}{169.93} \times 100 \approx 5.57\%
Final Answers:
- [Fe²⁺] = 0.1605 mol/L
- Molar mass = 179.4 g/mol
- Closest match: FeSO₄·4H₂O
- Percent error = 5.57%
