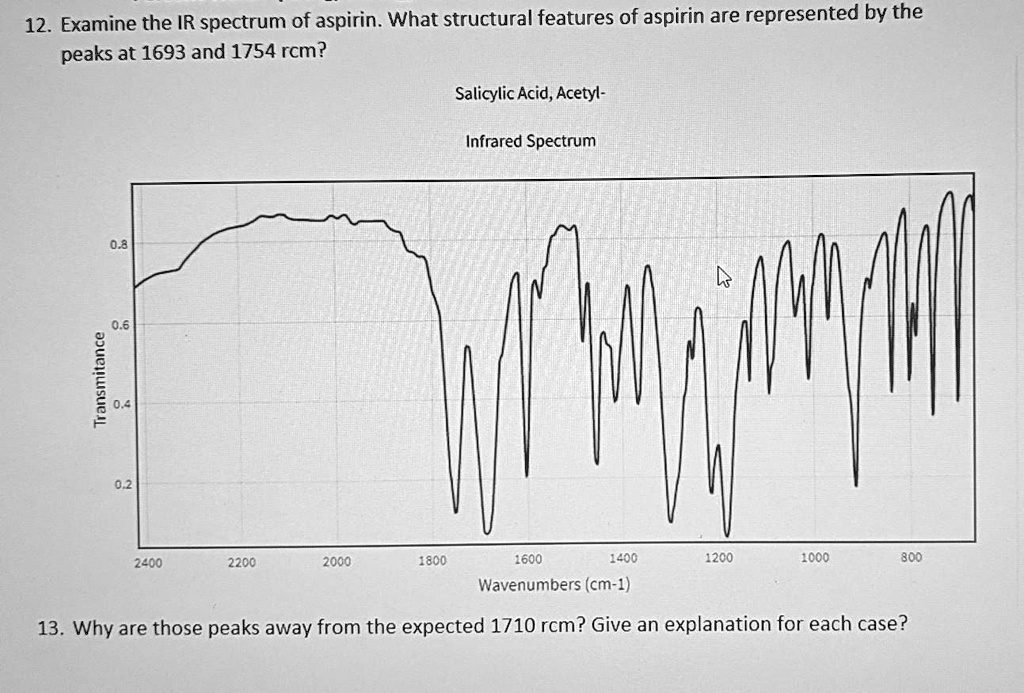
Examine the IR spectrum of aspirin. What structural features of aspirin are represented by the peaks at 1693 and 1754 rcm? Salicylic Acid; Acetyl- Infrared Spectrum M L 0.2 2400 2200 2000 1800 1600 1200 1200 1000 800 Wavenumbers (cm-1) 13. Why are those peaks away from the expected 1710 rcm? Give an explanation for each case?
The Correct Answer and Explanation is:
Question 12:
The peaks at 1693 cm⁻¹ and 1754 cm⁻¹ in the IR spectrum of aspirin correspond to the carbonyl (C=O) stretching vibrations of two distinct functional groups in the molecule. The 1693 cm⁻¹ peak represents the ester carbonyl group formed when the hydroxyl of salicylic acid reacts with acetic anhydride, yielding the acetylated form of aspirin. The 1754 cm⁻¹ peak corresponds to the carboxylic acid carbonyl group that remains from the original salicylic acid structure.
Question 13:
These observed C=O stretching frequencies deviate from the expected value of 1710 cm⁻¹, and this can be explained by the surrounding electronic environments and intermolecular interactions.
The 1693 cm⁻¹ ester peak appears at a lower wavenumber because of conjugation with the adjacent aromatic ring. This conjugation spreads electron density across the system, decreasing the bond order of the carbonyl group, which in turn reduces the force constant for vibration. A weaker C=O bond requires less energy to stretch, so the vibration is shifted to a lower wavenumber.
On the other hand, the 1754 cm⁻¹ carboxylic acid peak appears at a higher wavenumber due to limited conjugation and the impact of intramolecular hydrogen bonding. In aspirin, the hydroxyl group of the carboxylic acid may form a hydrogen bond with a nearby electronegative oxygen, stabilizing the electron distribution and stiffening the carbonyl bond. This reinforcement leads to a stronger C=O stretching vibration that occurs at a higher energy, causing the peak to shift upward.
These two shifts provide insight into the chemical structure of aspirin and the nature of its interactions, confirming that both an ester and a carboxylic acid group are retained in the molecule. The precise positioning of these bands helps distinguish aspirin from its precursors and related compounds.
