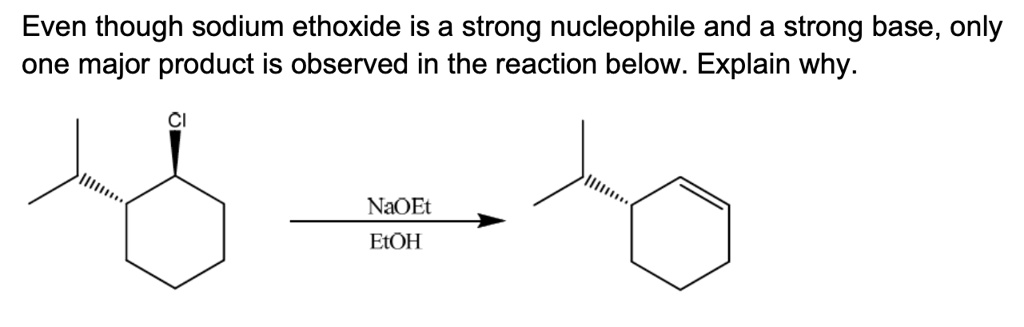
Even though sodium ethoxide is a strong nucleophile and a strong base, only one major product is observed in the reaction below. Explain why.
The Correct Answer and Explanation is:
The reaction proceeds via an E2 elimination mechanism, which has a strict stereochemical requirement. For the reaction to occur, the leaving group (chlorine) and a hydrogen atom on an adjacent carbon (a beta-hydrogen) must be in an anti-periplanar, or trans-diaxial, arrangement. This geometric constraint dictates which product can be formed.
The starting material, a trans-1,2-disubstituted cyclohexane, exists as an equilibrium between two chair conformations. The most stable conformation places the bulky isopropyl group in the equatorial position to minimize steric strain. In this conformation, the chlorine atom is also equatorial. Since the E2 reaction requires an axial leaving group, no reaction can occur from this major, more stable conformer.
The reaction must therefore proceed through the less stable chair conformation, where both the isopropyl group and the chlorine atom are in axial positions. Although this conformer is present in a very small amount at equilibrium, it is the only one that is reactive.
Once the molecule is in this reactive trans-diaxial conformation, the ethoxide base can remove a beta-hydrogen. There are two beta-carbons, at positions 2 and 6.
- At carbon 2: The isopropyl group is in the axial position. This means the hydrogen on carbon 2 is in the equatorial position. It is not anti-periplanar to the axial chlorine, so elimination cannot occur toward this carbon.
- At carbon 6: This carbon has two hydrogens. One is axial and one is equatorial. The axial hydrogen is perfectly positioned anti-periplanar to the axial chlorine atom.
Because only the axial hydrogen on carbon 6 satisfies the geometric requirement for E2 elimination, the ethoxide base can only abstract this specific proton. This leads exclusively to the formation of a double bond between carbons 1 and 6, resulting in 3-isopropylcyclohex-1-ene as the single major product. The SN2 pathway is disfavored due to the strong basicity of ethoxide and the steric hindrance at the secondary carbon
