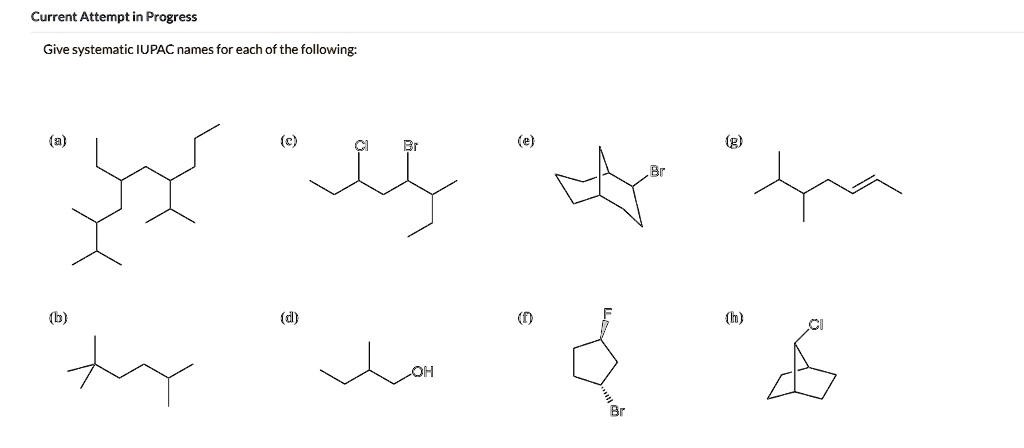
Current Attempt in Progress Give systematic IUPAC names for each of the following: Oh
The Correct Answer and Explanation is:
Systematic IUPAC Names:
(a) 2,3,4-Trimethylhexane
(b) 3-Methylpentane
(c) 2-Bromo-3-chlorohexane
(d) 2-Pentanol
(e) 2-Bromobicyclo[2.2.1]heptane
(f) trans-1-Bromo-2-fluorocyclopentane
(g) 4-Methyl-1-hexene
(h) 1-Chlorobicyclo[2.2.1]heptane
Explanation (about 300 words):
Naming organic compounds using IUPAC rules requires careful attention to the structure, substituents, and functional groups.
(a) The longest chain has 6 carbon atoms. There are three methyl groups on carbons 2, 3, and 4. So, the name is 2,3,4-trimethylhexane.
(b) The longest continuous chain has 5 carbons (pentane), with a methyl group on carbon 3. Thus, this is 3-methylpentane.
(c) The chain has 6 carbon atoms. A chlorine is on carbon 3, and a bromine on carbon 2. Numbering from the left gives the lowest set of locants. Hence, 2-bromo-3-chlorohexane.
(d) This is a five-carbon chain (pentane) with a hydroxyl group (alcohol) on carbon 2. So, it becomes 2-pentanol. The alcohol takes priority in numbering.
(e) This compound is a bicyclic molecule resembling norbornane. A bromine atom is attached to position 2. The name is 2-bromobicyclo[2.2.1]heptane, based on IUPAC rules for bicycloalkanes.
(f) A five-membered ring has bromine and fluorine attached to adjacent carbons. Since the substituents are on opposite sides of the ring (one wedge, one dash), it is trans-1-bromo-2-fluorocyclopentane.
(g) The longest chain has six carbons, with a double bond starting at carbon 1 and a methyl group on carbon 4. The correct name is 4-methyl-1-hexene.
(h) This is also a norbornane structure with a chlorine at bridgehead position 1. The IUPAC name is 1-chlorobicyclo[2.2.1]heptane.
Each name follows the rules of identifying the parent chain or ring, correctly numbering the carbons, and listing substituents alphabetically with appropriate locants.
