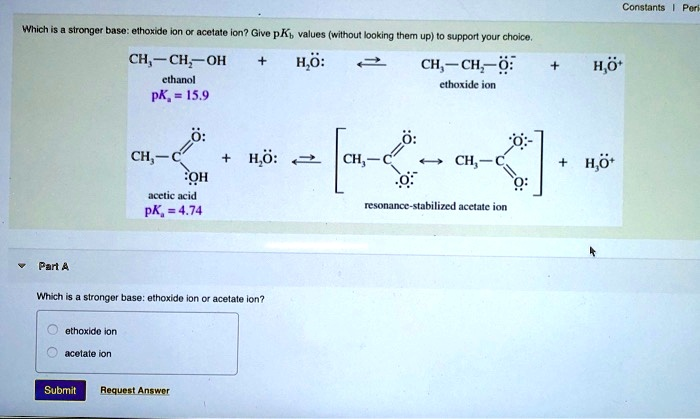
Constants Ethoxide ion accelerates ion? How – pKb values (without looking them up) support your choice. CH3CH2OH ethanol pK = 15.9 H2O: CH3CH2O- ethoxide ion 4.7 CH3CH2COOH acetic acid pK = 4.74 H2O: CH3CH2COO- resonance-stabilized acetate ion Which is a stronger base: ethoxide ion or acetic ion? Ethoxide ion Acetate ion
The Correct Answer and Explanation is:
The stronger base between ethoxide ion and acetate ion is the ethoxide ion.
To determine this, we compare the conjugate acids of the two ions: ethanol (CH₃CH₂OH) for ethoxide and acetic acid (CH₃COOH) for acetate. The strength of a base is inversely related to the strength of its conjugate acid. That means the weaker the conjugate acid, the stronger the base.
Ethanol has a pKa of 15.9 while acetic acid has a pKa of 4.74. A higher pKa value indicates a weaker acid. Since ethanol is a weaker acid than acetic acid, its conjugate base, the ethoxide ion (CH₃CH₂O⁻), is the stronger base when compared to the acetate ion (CH₃COO⁻).
This conclusion can also be supported by considering the stability of the conjugate bases. Acetate ion benefits from resonance stabilization. The negative charge on the oxygen is delocalized across two oxygen atoms, making the ion more stable and therefore less reactive. A more stable base is less likely to accept a proton, meaning it is weaker in terms of basicity.
On the other hand, the ethoxide ion does not have resonance stabilization. Its negative charge is localized on a single oxygen atom, making it more eager to accept a proton. This increases its basicity, making ethoxide a stronger base.
Therefore, based on the pKa values of their conjugate acids and the structural stability of the anions, ethoxide ion is definitively the stronger base compared to acetate ion. This conclusion helps explain the behavior of these species in acid-base reactions, especially in organic synthesis where ethoxide is frequently used as a strong base.
