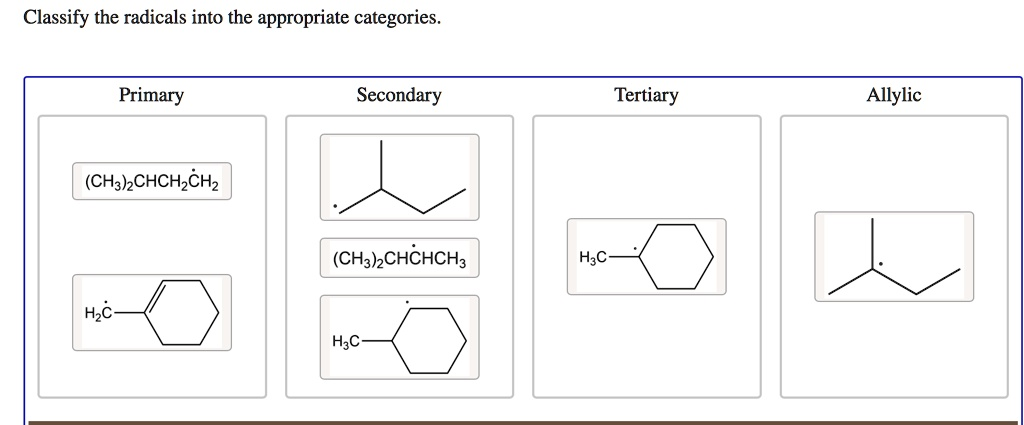
Classify the radicals into the appropriate categories: Primary Secondary Tertiary Allylic (CH3)CH2CH2CH2 (CH3)CHCH3 H2C HC HC
The Correct Answer and Explanation is:
Based on chemical principles, here is the correct classification of the radicals:
Primary
- (CH₃)₂CHCH₂ĊH₂
Secondary
- The skeletal structure of the pentan-2-yl radical
- (CH₃)₂CHĊHCH₃
- The skeletal structure of the methylcyclohexyl radical
Tertiary
- The skeletal structure of the 1-methylcyclohex-1-yl radical
- The skeletal structure of the 2-methylbutan-2-yl radical (incorrectly placed in the “Allylic” category in the image)
Allylic
- The skeletal structure of the (cyclohex-1-en-1-yl)methyl radical (incorrectly placed in the “Primary” category in the image)
Explanation
The classification of carbon radicals depends on the nature of the carbon atom that carries the unpaired electron, known as the radical center. The categories are defined as follows:
- A primary (1°) radical has the unpaired electron on a carbon atom that is bonded to only one other carbon atom.
- A secondary (2°) radical has the unpaired electron on a carbon atom that is bonded to two other carbon atoms.
- A tertiary (3°) radical has the unpaired electron on a carbon atom that is bonded to three other carbon atoms.
- An allylic radical has the unpaired electron on a carbon atom that is adjacent to a carbon-carbon double bond (C=C). This position is significant because the radical is stabilized by resonance, meaning the unpaired electron can delocalize over the pi system of the double bond. An allylic radical is typically more stable than a simple alkyl radical of the same substitution (primary, secondary, or tertiary).
Analysis of Each Radical:
- (CH₃)₂CHCH₂ĊH₂: The radical center is the terminal ĊH₂ group. This carbon is directly bonded to only one other carbon atom. Therefore, it is correctly classified as a primary radical.
- Pentan-2-yl radical (skeletal structure): The radical center is a CH group within the carbon chain. This carbon is bonded to two other carbons, one on each side. This makes it a secondary radical.
- (CH₃)₂CHĊHCH₃: The radical center is the ĊH group. This carbon is bonded to two other carbons: the carbon of the isopropyl group and the carbon of the methyl group. This is a secondary radical.
- Methylcyclohexyl radical (skeletal structure): The radical center is a carbon atom within the cyclohexane ring. This carbon is bonded to two other carbon atoms as part of the ring structure. The methyl group is attached to a different carbon. This is a secondary radical.
- 1-methylcyclohex-1-yl radical (skeletal structure): The radical center is the ring carbon that is also bonded to the methyl group. This carbon atom is attached to three other carbons: two within the ring and one in the methyl group. This is correctly classified as a tertiary radical.
Corrections to the Provided Image:
The classification shown in the original image contains two errors:
- (Cyclohex-1-en-1-yl)methyl radical (skeletal structure): This radical is shown in the “Primary” box. While its radical center (H₂Ċ) is bonded to only one carbon, making it technically primary, its most important structural feature is its position next to the C=C double bond in the ring. This proximity allows for resonance stabilization, which makes it an allylic radical. It should be in the “Allylic” category.
- 2-methylbutan-2-yl radical (skeletal structure): This radical is shown in the “Allylic” box. Its radical center is a carbon atom bonded to three other carbons (two methyl groups and one ethyl group). It does not contain any double bonds, so it cannot be allylic. It is a tertiary radical and should be placed in the “Tertiary” category.
