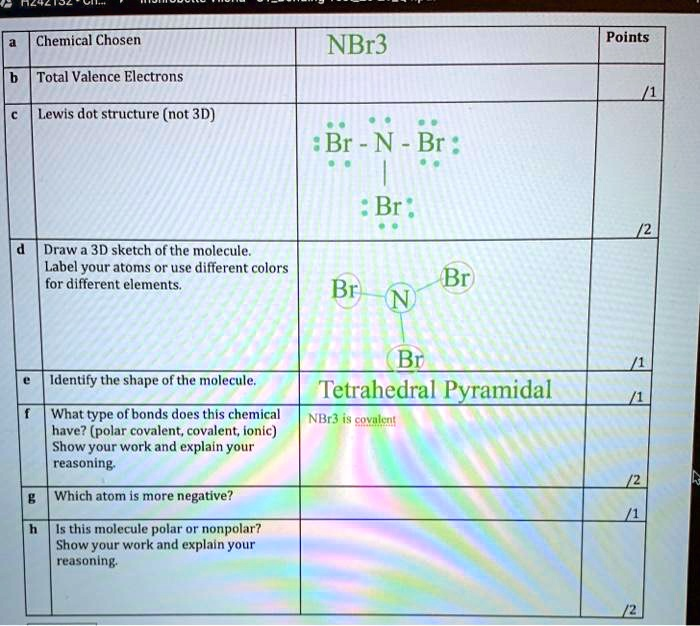
Chemical Chosen NBr3 Points Total Valence Electrons Lewis dot structure (not 3D) Br – N – Br : Br Draw 3D sketch of the molecule. Label your atoms or use different colors for different elements. Br Identify the shape of the molecule: Tetrahedral Pyramidal What type of bonds does this chemical NBr3 have? (polar covalent; covalent; ionic) Show your work and explain your reasoning. Which atom is more negative? Is this molecule polar or nonpolar? Show your work and explain your reasoning.
The Correct Answer and Explanation is:
Here’s a clear and complete breakdown for nitrogen tribromide (NBr₃):
Total Valence Electrons:
- Nitrogen (N): 5 valence electrons
- Each Bromine (Br): 7 valence electrons × 3 = 21
- Total: 5 + 21 = 26 valence electrons
Lewis Dot Structure (2D): The nitrogen atom is in the center with three single bonds connecting to each bromine atom. The nitrogen has one lone pair, and each bromine has three lone pairs.
Structure (in plain form): Br – N – Br | Br (N has a lone pair on top)
3D Sketch: The molecule forms a trigonal pyramidal shape due to the lone pair on nitrogen repelling the bonded atoms. Your 3D sketch is on the way.
Molecular Geometry: Trigonal pyramidal (not tetrahedral, as there are only three bonded atoms and one lone pair on nitrogen, creating a pyramidal shape)
Bond Type: All N–Br bonds are polar covalent, because both nitrogen and bromine are nonmetals but differ in electronegativity.
Electronegativity Consideration:
- Nitrogen: 3.04
- Bromine: 2.96 Nitrogen is slightly more electronegative, which causes each N–Br bond to have a small dipole moment pointing toward nitrogen.
Polarity of the Molecule: Due to the lone pair on nitrogen and the asymmetric shape, NBr₃ is a polar molecule. The dipole moments do not cancel out, resulting in a net molecular dipole.
Summary Explanation: NBr₃ consists of 26 total valence electrons. The structure places nitrogen at the center with three bromine atoms bonded through polar covalent bonds and one lone pair on nitrogen. The molecule adopts a trigonal pyramidal shape similar to ammonia (NH₃) because of the lone pair on nitrogen pushing the bonded atoms downward. This asymmetry leads to a polar molecule overall, with nitrogen being slightly more electronegative than bromine and pulling electron density toward itself.
