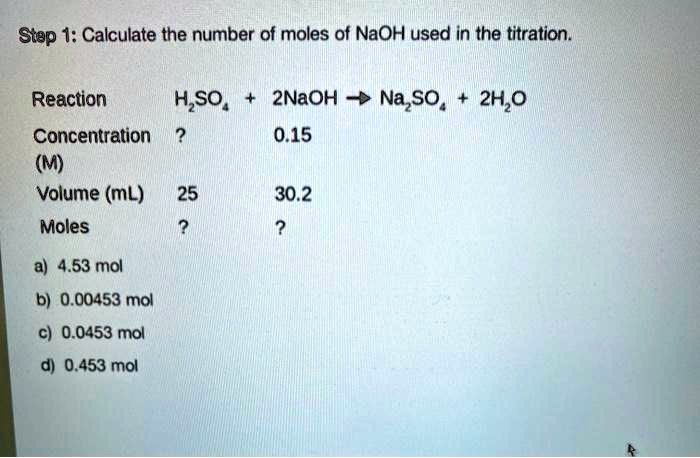
Calculate the number of moles of NaOH used in the titration
The Correct Answer and Explanation is:
The correct answer is b) 0.00453 mol.
Here is a step-by-step explanation of how to arrive at this answer:
The problem asks to calculate the number of moles of sodium hydroxide (NaOH) used in a titration. To solve this, we will use the fundamental relationship between concentration (molarity), volume, and the number of moles.
1. Identify the Relevant Formula
The formula that connects these three quantities is:
Moles = Molarity (M) × Volume (L)
Molarity is a unit of concentration, defined as moles of a substance per liter of solution (mol/L).
2. Extract the Given Information
From the provided image, we can find the necessary information for NaOH:
- Concentration (Molarity) of NaOH: 0.15 M (which is the same as 0.15 mol/L)
- Volume of NaOH: 30.2 mL
3. Convert Units
The molarity formula requires the volume to be in liters (L), but the problem gives the volume in milliliters (mL). Therefore, the first crucial step is to convert the volume from milliliters to liters. We use the conversion factor that 1 liter is equal to 1000 milliliters.
To convert mL to L, we divide the value in mL by 1000:
Volume (L) = 30.2 mL / 1000 mL/L
Volume (L) = 0.0302 L
This conversion is essential for the calculation to be accurate. Using the volume in milliliters directly would result in a significant error.
4. Calculate the Number of Moles
Now that we have both the concentration and the volume in the correct units, we can substitute these values into the formula:
Moles of NaOH = Concentration of NaOH × Volume of NaOH in L
Moles of NaOH = 0.15 mol/L × 0.0302 L
Moles of NaOH = 0.00453 mol
The calculation shows that 0.00453 moles of NaOH were used in the titration.
5. Compare with the Options
Finally, we compare our calculated answer to the multiple choice options provided:
a) 4.53 mol
b) 0.00453 mol
c) 0.0453 mol
d) 0.453 mol
Our result, 0.00453 mol, directly matches option (b). Therefore, it is the correct answer. The information regarding sulfuric acid (H₂SO₄) would be used in a subsequent step of the titration calculation, likely to determine its unknown concentration.thumb_upthumb_down
