Below is orbitals_ molecular orbital diagram of carbon monoxide, showing only the valence 2p 2p 25 2s
The Correct Answer and Explanation is:
Correct Answer: orbitals
Explanation
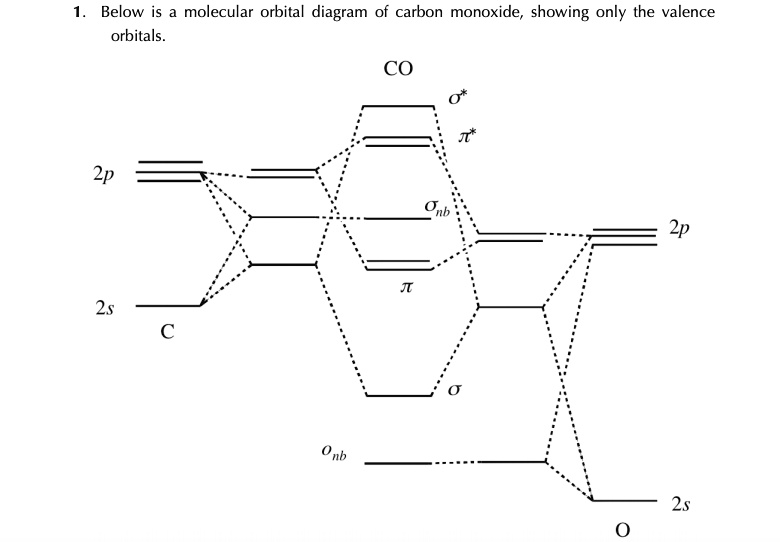
The provided image displays a molecular orbital (MO) diagram for carbon monoxide (CO), showing how the valence atomic orbitals of carbon and oxygen combine to form the molecule’s molecular orbitals.
The diagram is structured with the atomic orbitals (AOs) of carbon (C) on the left and oxygen (O) on the right, with the resulting molecular orbitals (MOs) for CO shown in the center. Oxygen is more electronegative than carbon, so its 2s and 2p atomic orbitals are lower in energy than carbon’s, as depicted in the diagram.
Carbon contributes 4 valence electrons (from its 2s and 2p orbitals), and oxygen contributes 6 (from its 2s and 2p orbitals), for a total of 10 valence electrons. These electrons fill the molecular orbitals starting from the lowest energy level, following the Aufbau principle and Hund’s rule.
The combination of the 2s and 2p atomic orbitals leads to the formation of bonding (σ, π), non-bonding (O_nb, σ_nb), and antibonding (π*, σ*) molecular orbitals. Due to the energy difference between the carbon and oxygen AOs, significant s-p mixing occurs. The 10 valence electrons fill the orbitals as follows: (O_nb)², (σ)², (π)⁴, (σ_nb)².
The bond order can be calculated as ½ (bonding electrons – antibonding electrons). In this diagram, the σ and π orbitals are bonding (6 electrons total), while the π* and σ* orbitals are antibonding (0 electrons). The O_nb and σ_nb are non-bonding. Thus, the bond order is ½ (6 – 0) = 3. This corresponds to the triple bond (C≡O) seen in the Lewis structure of carbon monoxide.
A key feature is that the Highest Occupied Molecular Orbital (HOMO) is the σ_nb orbital, which is predominantly located on the carbon atom. This explains why carbon monoxide typically acts as a ligand and bonds to metal atoms through its carbon end. The Lowest Unoccupied Molecular Orbital (LUMO) is the degenerate pair of π* orbitals.
