BeCl2 Molecular Geometry and VSEPR Theory
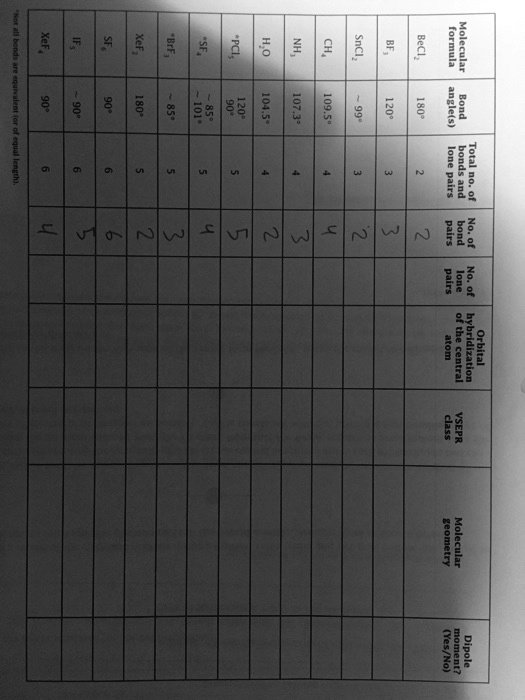
The Correct Answer and Explanation is:
The molecular geometry of beryllium chloride (BeCl₂) is linear.
Beryllium chloride consists of a central beryllium atom bonded to two chlorine atoms. According to Valence Shell Electron Pair Repulsion (VSEPR) theory, the geometry of a molecule is determined by the number of bonding and lone electron pairs around the central atom. In the case of BeCl₂, beryllium has two valence electrons and forms two single covalent bonds with chlorine atoms. It does not retain any lone pairs after bonding.
This gives BeCl₂ an AX₂ VSEPR classification. “A” represents the central atom, and “X” represents the number of atoms bonded to it. Since there are no lone pairs, the molecule adopts a configuration that minimizes repulsion between the bonding pairs. The only arrangement that achieves this in three-dimensional space is a straight line, resulting in a bond angle of 180 degrees.
To form these bonds, beryllium undergoes sp hybridization. This involves the mixing of one 2s and one 2p orbital to form two equivalent sp hybrid orbitals. Each of these orbitals forms a sigma bond with a chlorine atom. The resulting structure is symmetrical and nonpolar because the dipole moments of the Be–Cl bonds cancel each other out.
Although beryllium is a metal and does not follow the octet rule, its bonding in BeCl₂ is still stable due to the small size and high charge density of the Be²⁺ ion, which allows for strong covalent interactions with the electronegative chlorine atoms.
In summary, BeCl₂ is an sp hybridized molecule with a linear shape, 180-degree bond angles, no lone pairs on the central atom, and no net dipole moment. VSEPR theory accurately predicts this structure by focusing on electron pair repulsion and spatial arrangement.
