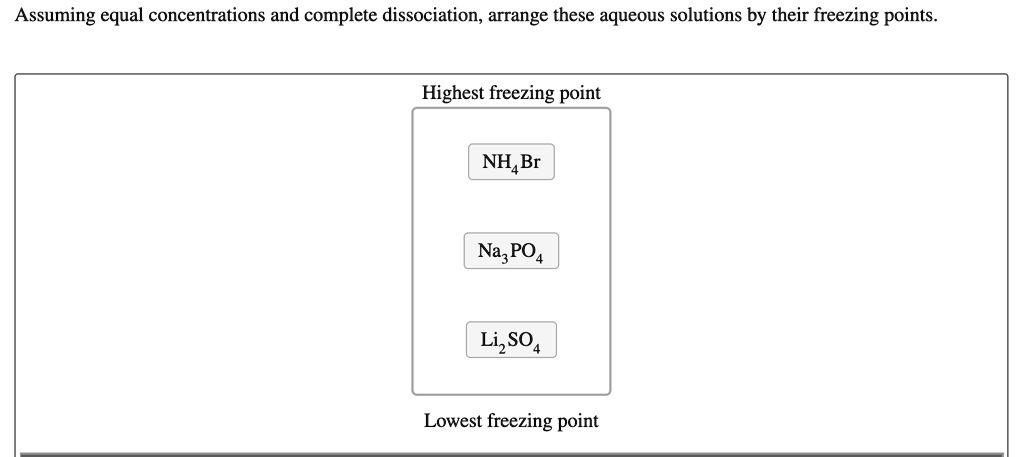
Assuming equal concentrations and complete dissociation, arrange these aqueous solutions by their freezing points_ Highest freezing point NHABr Na;” PO4 LizSO Lowest freezing point
The Correct Answer and Explanation is:
To determine the correct order of freezing points for the aqueous solutions given, we need to understand freezing point depression, a colligative property. This property depends on the number of particles the solute produces in solution and not on their identity. The more particles present in solution, the lower the freezing point will be.
Each compound dissociates in water as follows:
- NH₄Br → NH₄⁺ + Br⁻ (2 ions)
- Na₃PO₄ → 3Na⁺ + PO₄³⁻ (4 ions)
- Li₂SO₄ → 2Li⁺ + SO₄²⁻ (3 ions)
Now, we apply the concept of van’t Hoff factor (i) which represents the number of particles into which a compound dissociates in solution:
- NH₄Br has i = 2
- Li₂SO₄ has i = 3
- Na₃PO₄ has i = 4
According to the formula for freezing point depression: ΔTf=i⋅Kf⋅m\Delta T_f = i \cdot K_f \cdot mΔTf=i⋅Kf⋅m
Where:
- ΔTₓ is the freezing point depression
- i is the van’t Hoff factor
- Kf is the cryoscopic constant (same for all as water is the solvent)
- m is the molality (same for all solutions as given)
Therefore, the more ions a solute produces, the lower the freezing point of the solution.
Conclusion:
- NH₄Br produces the fewest particles, so it will have the highest freezing point
- Li₂SO₄ produces more particles than NH₄Br but fewer than Na₃PO₄, so it is in the middle
- Na₃PO₄ produces the most particles, leading to the lowest freezing point
Correct Order:
Highest freezing point: NH₄Br
Middle: Li₂SO₄
Lowest freezing point: Na₃PO₄
