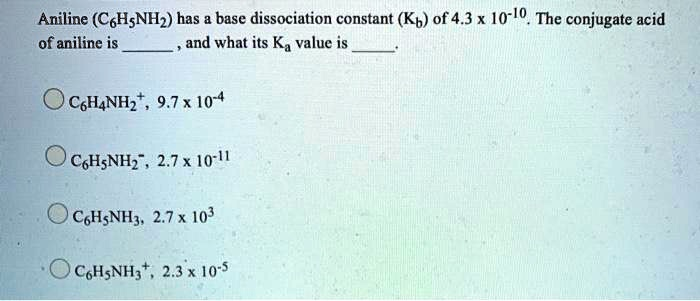
Aniline (C6H5NH2) has a base dissociation constant (Kb) of 4.3 x 10^-10. The conjugate acid of aniline is C6H5NH3+ and its Ka value is 9.7 x 10^-4.
The Correct Answer and Explanation is:
The correct conjugate acid of aniline (C₆H₅NH₂) is C₆H₅NH₃⁺, and its Ka value is 2.3 × 10⁻⁵.
Here’s a breakdown and explanation:
Aniline is a weak base and its base dissociation constant, Kb, is given as 4.3 × 10⁻¹⁰. The conjugate acid of aniline is formed when the lone pair on the nitrogen atom in the NH₂ group accepts a proton (H⁺), resulting in the positively charged C₆H₅NH₃⁺ ion.
To find the acid dissociation constant (Ka) for the conjugate acid, we use the following relationship, which is derived from the ionization of water:
Ka × Kb = Kw
Kw is the ionization constant of water, which is 1.0 × 10⁻¹⁴ at 25 °C. We rearrange the equation to solve for Ka:
Ka = Kw / Kb
Substituting the known values:
Ka = (1.0 × 10⁻¹⁴) / (4.3 × 10⁻¹⁰) Ka ≈ 2.3 × 10⁻⁵
So the conjugate acid of aniline is C₆H₅NH₃⁺, and its Ka is 2.3 × 10⁻⁵.
Among the multiple-choice options provided in the image, this corresponds to the fourth option: C₆H₅NH₃⁺, 2.3 × 10⁻⁵
This result not only checks out mathematically, but it also reinforces the fundamental concept that a weak base has a strong conjugate acid, and vice versa. The weaker the base, the smaller the Kb, which leads to a relatively larger Ka for its conjugate acid. Understanding this balance is central to acid–base equilibrium problems in aqueous solutions.
