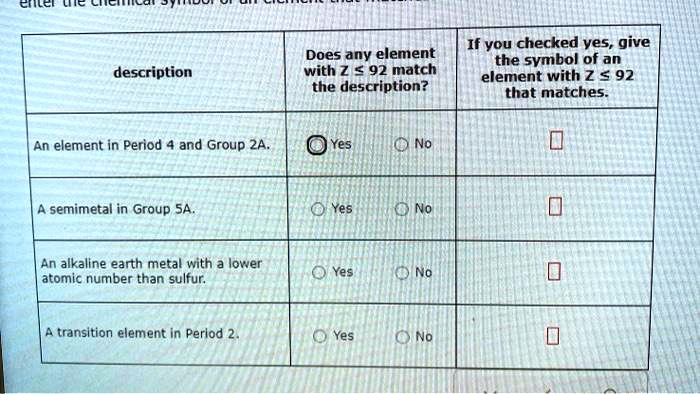
If you checked yes, give the symbol of an element with Z < 92 that matches. Does any element with Z < 92 match the description? Description: An element in Period and Group 2A. Yes. No. Semimetal in Group 5A. Yes. No. An alkaline earth metal with lower atomic number than sulfur: Yes. No. Transition element in Period 2. Yes.
The Correct Answer and Explanation is:
Here are the correct answers for each item along with a detailed explanation:
- An element in Period 4 and Group 2A Answer: Yes Symbol: Ca Calcium (Ca), with atomic number 20, is located in Period 4 and Group 2A of the periodic table. Group 2A elements are alkaline earth metals and include beryllium, magnesium, calcium, strontium, barium, and radium. Calcium is well known for its biological significance, especially in bone structure and cellular functions.
- A semimetal in Group 5A Answer: Yes Symbol: As Arsenic (As), with atomic number 33, is a classic example of a semimetal or metalloid. It belongs to Group 5A, also known as Group 15, which contains nitrogen, phosphorus, arsenic, antimony, and bismuth. Arsenic has properties intermediate between metals and nonmetals and is used in semiconductors and as a pesticide.
- An alkaline earth metal with a lower atomic number than sulfur Answer: Yes Symbol: Mg Magnesium (Mg), atomic number 12, qualifies as an alkaline earth metal with a lower atomic number than sulfur, which has atomic number 16. Magnesium is highly reactive and essential for many biological processes including ATP metabolism.
- A transition element in Period 2 Answer: No There are no transition metals in Period 2. Period 2 consists of elements from lithium (Z = 3) to neon (Z = 10), none of which fall in the d-block of the periodic table. Transition metals begin in Period 4 with elements like scandium and titanium.
These responses are consistent with the rules of periodic classification. The periodic table organizes elements by increasing atomic number into periods (rows) and groups (columns), each reflecting recurring chemical properties. The elements listed above exemplify how specific attributes align with well-defined groups
