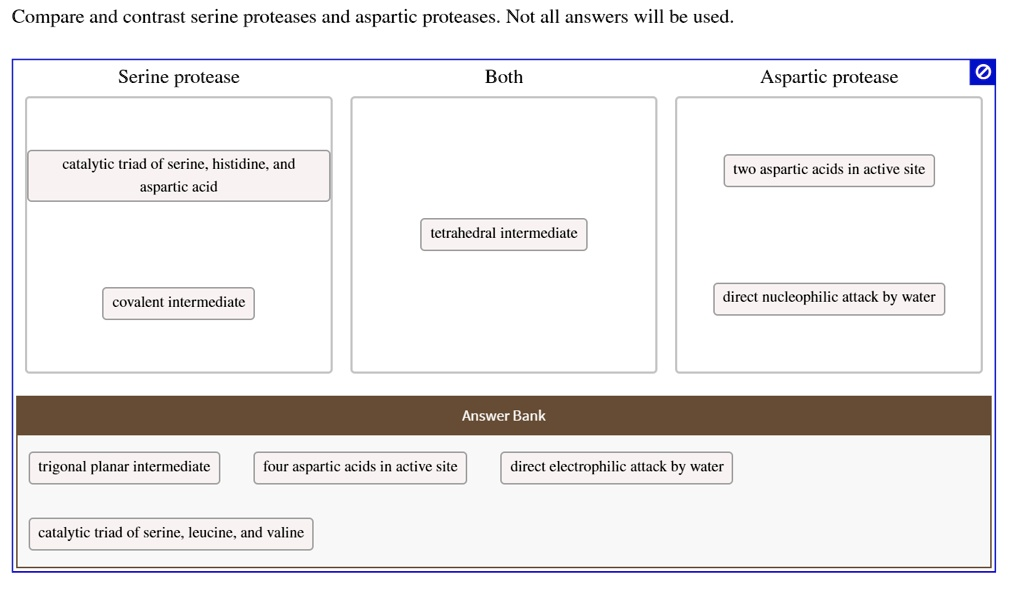
Compare and contrast serine proteases and aspartic proteases. Not all answers will be used: Serine protease: – Catalytic triad of serine, histidine, and aspartic acid – Tetrahedral intermediate – Covalent intermediate – Direct nucleophilic attack by water Aspartic protease: – Four aspartic acids in active site – Trigonal planar intermediate – Direct electrophilic attack by water
The Correct Answer and Explanation is:
Correct Answer
Here is the correct categorization for the terms comparing serine and aspartic proteases:
- Serine protease:
- catalytic triad of serine, histidine, and aspartic acid
- covalent intermediate
- Both:
- tetrahedral intermediate
- Aspartic protease:
- two aspartic acids in active site
- direct nucleophilic attack by water
Explanation
Serine proteases and aspartic proteases are two major classes of enzymes that catalyze the hydrolysis of peptide bonds, but they employ distinct catalytic mechanisms.
A defining characteristic of serine proteases is the presence of a catalytic triad in their active site, composed of serine, histidine, and aspartic acid residues. In this mechanism, the histidine residue acts as a general base to activate the serine’s hydroxyl group, making it a powerful nucleophile. This activated serine then attacks the carbonyl carbon of the substrate’s peptide bond. A key step in this process is the formation of a covalent intermediate, specifically an acyl-enzyme intermediate, where the substrate is temporarily bonded to the enzyme’s serine residue.
In contrast, aspartic proteases have an active site containing a pair of two aspartic acid residues. These two residues work together to activate a water molecule. One aspartate, acting as a general base, deprotonates the water, making it a strong nucleophile. This activated water molecule then performs a direct nucleophilic attack by water on the peptide bond’s carbonyl carbon. Unlike serine proteases, this mechanism does not involve the formation of a covalent enzyme-substrate intermediate.
Despite these significant differences in generating the nucleophile, both enzyme classes share a common feature in their reaction pathway. The nucleophilic attack on the carbonyl carbon, whether by serine or by water, results in the formation of a high-energy tetrahedral intermediate. This transient state, where the carbonyl carbon changes from a planar to a tetrahedral geometry, is a fundamental aspect of the peptide bond cleavage catalyzed by both serine and aspartic proteases. The stabilization of this intermediate is critical for lowering the activation energy of the reaction. The other options in the answer bank are incorrect distractors.
