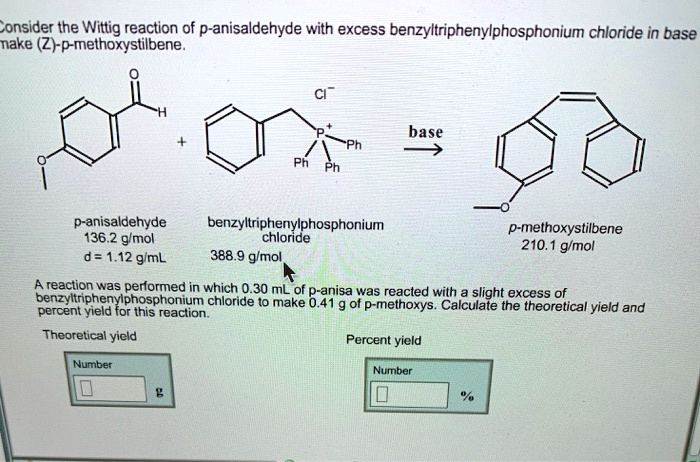
Consider the Wittig reaction of p-anisaldehyde with excess benzyltriphenylphosphonium chloride in base make (Z)-p-methoxystilbene. CI H +
Ph Ph Ph base ? p-anisaldehyde 136.2 g/mol d = 1.12 g/mL benzyltriphenylphosphonium chloride 388.9 g/mol p-methoxystilbene 210.1 g/mol A reaction was performed in which 0.30 mL of p-anisa was reacted with a slight excess of benzyltriphenylphosphonium chloride to make 0.41 g of p-methoxys. Calculate the theoretical yield and percent yield for this reaction. Theoretical yield Number g Percent yield Number %
The Correct Answer and Explanation is:
Theoretical Yield: 0.52 g
Percent Yield: 79 %
Explanation
1. Identify the Limiting Reactant
The problem states that p-anisaldehyde is reacted with an “excess” of benzyltriphenylphosphonium chloride. This means that p-anisaldehyde is the limiting reactant, and it will be completely consumed first. The maximum amount of product that can be formed (the theoretical yield) is determined by the starting amount of this limiting reactant.
2. Calculate the Theoretical Yield
The calculation follows these steps:
- Step A: Find the mass of p-anisaldehyde.
We are given the volume (0.30 mL) and density (1.12 g/mL) of p-anisaldehyde.
Mass = Volume × Density
Mass = 0.30 mL × 1.12 g/mL = 0.336 g - Step B: Convert the mass of p-anisaldehyde to moles.
Using its molar mass (136.2 g/mol ):
Moles = Mass / Molar Mass
Moles = 0.336 g / 136.2 g/mol = 0.002467 mol of p-anisaldehyde - Step C: Determine the moles of product (p-methoxystilbene).
The stoichiometry of the Wittig reaction between the aldehyde and the ylide to form the alkene is 1:1. Therefore, 0.002467 moles of p-anisaldehyde will theoretically produce 0.002467 moles of p-methoxystilbene. - Step D: Convert the moles of product to grams (Theoretical Yield).
Using the molar mass of p-methoxystilbene (210.1 g/mol ):
Theoretical Yield (g) = Moles × Molar Mass
Theoretical Yield (g) = 0.002467 mol × 210.1 g/mol = 0.5183 g
Rounding to two significant figures (based on the 0.30 mL measurement), the theoretical yield is 0.52 g.
3. Calculate the Percent Yield
The percent yield compares the actual amount of product obtained with the maximum possible amount (the theoretical yield).
- Formula: Percent Yield = (Actual Yield / Theoretical Yield) × 100%
- Given Actual Yield: 0.41 g
- Calculated Theoretical Yield: 0.5183 g (using the unrounded value for accuracy)
Percent Yield = (0.41 g / 0.5183 g) × 100% = 79.1%
Rounding to two significant figures (based on the 0.41 g measurement), the percent yield is 79 %.
