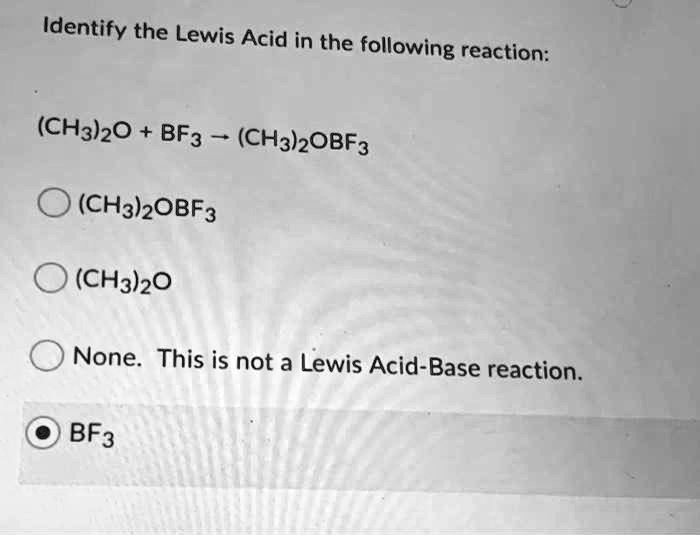
Identify the Lewis Acid in the following reaction: (CH3)2O + BF3 -> (CH3)OBF3 + (CH3)2O (CH3)OBF3 (CH3)2O None – This is not a Lewis Acid-Base reaction. BF3
The Correct Answer and Explanation is:
The correct answer is BF₃.
Explanation:
This reaction is a classic example of a Lewis acid base interaction. To identify the Lewis acid, we first need to understand the definitions of Lewis acids and bases. A Lewis acid is a chemical species that acts as an electron pair acceptor. Conversely, a Lewis base is a species that acts as an electron pair donor. The reaction involves the formation of a new covalent bond, known as a coordinate covalent bond, where one species provides both electrons for the bond.
Let’s analyze the reactants in the given equation: (CH₃)₂O + BF₃ → (CH₃)₂OBF₃.
First, consider dimethyl ether, (CH₃)₂O. The central oxygen atom is bonded to two methyl groups. According to its position in the periodic table (Group 16), oxygen has six valence electrons. It uses two of these electrons to form single bonds with the carbon atoms of the methyl groups. The remaining four valence electrons exist as two non-bonding lone pairs. The presence of these available lone pairs makes dimethyl ether capable of donating a pair of electrons. Therefore, (CH₃)₂O acts as the Lewis base.
Next, let’s examine boron trifluoride, BF₃. The central atom is boron, which is in Group 13 and has three valence electrons. It forms three single covalent bonds with three fluorine atoms. In the BF₃ molecule, the boron atom is surrounded by only six electrons (three bonds × two electrons each), leaving it with an incomplete valence shell or octet. This electron deficiency creates an empty p-orbital on the boron atom, making it highly receptive to accepting a pair of electrons to achieve a stable electron configuration. Consequently, BF₃ acts as the Lewis acid.
In the reaction, the oxygen atom of dimethyl ether donates one of its lone pairs to the electron deficient boron atom of boron trifluoride. This forms a coordinate covalent bond between oxygen and boron, resulting in the product or adduct, (CH₃)₂OBF₃.
Since BF₃ is the species that accepts the electron pair, it is correctly identified as the Lewis acid in this reaction
